10-K: Annual report pursuant to Section 13 and 15(d)
Published on March 14, 2024
c
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended
OR
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO |
Commission File Number
(Exact name of Registrant as specified in its Charter)
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|
|
|
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
None |
N/A |
N/A |
Securities registered pursuant to Section 12(g) of the Act:
(Title of class)
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
|
☐ |
|
|
☒ |
|
|
|
|
|
|||
Non-accelerated filer |
|
☐ |
|
Smaller reporting company |
|
|
|
|
|
|
|
|
|
Emerging growth company |
|
|
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant's executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No
The aggregate market value of the voting and non-voting common equity (on an as-converted basis, based on the closing price of these shares on the Canadian Securities Exchange (the "CSE")) on June 30, 2023, the last business day of the Registrant’s most recently completed second fiscal quarter, held by non-affiliates of the Registrant was $
The number of shares of Registrant’s Common Share (as defined below) outstanding as of March 14, 2024 was
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive Proxy Statement (the "Proxy Statement" relating to the 2024 Annual Meeting of Shareholders (the “Annual Meeting”) are incorporated by reference into Part III of this Annual Report on Form 10-K (this “Annual Report”) where indicated. The Proxy Statement will be filed with the United States Securities and Exchange Commission (the "SEC") within 120 of the Registrant’s fiscal year ended December 31, 2023.
Table of Contents
|
|
Page |
PART I |
|
7 |
Item 1. |
7 |
|
Item 1A. |
Risk Factors |
25 |
Item 1B. |
Unresolved Staff Comments |
52 |
Item 1C. |
53 |
|
Item 2. |
Properties |
54 |
Item 3. |
Legal Proceedings |
59 |
Item 4. |
Mine Safety Disclosures |
59 |
|
|
|
PART II |
|
60 |
Item 5. |
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
60 |
Item 6. |
[Reserved] |
62 |
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
63 |
Item 7A. |
83 |
|
Item 8. |
Financial Statements and Supplementary Data |
84 |
Item 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
84 |
Item 9A. |
Controls and Procedures |
84 |
Item 9B. |
Other Information |
85 |
Item 9C. |
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
85 |
|
|
|
PART III |
|
86 |
Item 10. |
Directors, Executive Officers and Corporate Governance |
86 |
Item 11. |
Executive Compensation |
86 |
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
86 |
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
86 |
Item 14. |
Principal Accounting Fees and Services |
86 |
|
|
|
PART IV |
|
87 |
Item 15. |
Exhibits, Financial Statement Schedules |
87 |
Item 16. |
Form 10-K Summary |
95 |
|
Signatures |
97 |
|
F-1 |
i
Cautionary Note Regarding Forward-Looking Statements
This Annual Report contains statements that TerrAscend Corp. (the "Issuer") believes are, or may be considered to be, “forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended (the "Securities Act"), and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"). All statements other than statements of historical fact included in this Annual Report regarding the prospects of the industry in which the Issuer, its subsidiaries, TerrAscend Growth Corp. (“TerrAscend”) and its subsidiaries (collectively, the "Company”) operate or the Company’s prospects, plans, financial position or business strategy may constitute forward-looking statements. Such statements can be identified by the use of forward-looking terminology such as "can", “expect”, “likely”, “may”, “will”, “should”, “intend”, “anticipate”, “potential”, “proposed”, “estimate” and other similar words, including negative and grammatical variations thereof, or statements that certain events or conditions “may” or “will” happen, or by discussions of strategy. Forward-looking statements include estimates, plans, expectations, opinions, forecasts, projections, targets, guidance, or other statements that are not statements of fact. Forward-looking statements in this Annual Report include, but are not limited to, statements with respect to:
1
Certain of the forward-looking statements contained herein concerning the cannabis industry and the general expectations of the Company concerning the cannabis industry are based on estimates prepared by the Company using data from publicly-available governmental sources as well as from market research and industry analysis and on assumptions based on data and knowledge of the cannabis industry. Such data is inherently imprecise. The cannabis industry involves risks and uncertainties that are subject to change based on various factors, which factors are described further below.
With respect to the forward-looking statements contained in this Annual Report, the Company has made assumptions regarding, among other things: (i) its ability to generate cash flows from operations and obtain necessary financing on acceptable terms; (ii) general economic, financial market, regulatory and political conditions in jurisdictions in which the Company operates; (iii) the output from the Company’s operations; (iv) consumer interest in the Company’s products; (v) competition in the cannabis industry; (vi) anticipated and unanticipated costs; (vii) government regulation of the Company’s activities and products; (viii) government regulation of licensing, taxation and environmental protection; (ix) the timely receipt of any required regulatory approvals; (x) the Company’s ability to obtain qualified staff, equipment and services in a timely and cost efficient manner; (xi) the Company’s ability to conduct operations in a safe, efficient and effective manner; and (xii) the Company’s construction plans and timeframe for completion of such plans.
Readers are cautioned that the above list of cautionary statements is not exhaustive. Known and unknown risks, many of which are beyond the control of the Company, could cause actual results to differ materially from the forward-looking statements in this Annual Report. Such risks and uncertainties include, but are not limited to, current and future market conditions; risks related to federal, state, provincial, territorial, local and foreign government laws, rules and regulations, including federal and state laws in the United States relating to cannabis operations in the United States; and those discussed under Item 1A – “Risk Factors” in this Annual Report and in the "Risk Factor Summary" below. The purpose of forward-looking statements is to provide the reader with a description of management’s expectations, and such forward-looking statements may not be appropriate for any other purpose. You should not place undue reliance on forward-looking statements contained in this Annual Report. The Company can give no assurance that such expectations will prove to have been correct. Forward-looking statements contained herein are made as of the date of this Annual Report and are based on the beliefs, estimates, expectations and opinions of management on the date such forward-looking statements are made. The Company undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, estimates or opinions, future events or results or otherwise or to explain any material difference between subsequent actual events and such forward-looking statements, except as required by applicable law.
2
Risk Factor Summary
Investing in the Common Shares involves risks. You should carefully consider the risks described in Item 1A – “Risk Factors” beginning on page 26 of this Annual Report before deciding to invest in the Common Shares. If any of these risks actually occur, the Company’s business, financial condition and results of operations could likely be materially adversely affected. In such case, the trading price of the Common Shares may decline, and you may lose all or part of your investment. Set forth below is a summary of some of the principal risks that the Company faces:
3
4
5
6
PART I
ITEM 1. BUSINESS
Overview
The Company is a leading North American cannabis company. The Company has vertically integrated licensed operations in Pennsylvania, New Jersey, Michigan, Maryland and California. In addition, the Company has retail operations in Ontario, Canada with a majority-owned dispensary in Toronto, Ontario, Canada. Notwithstanding the fact that various states in the United States have implemented medical cannabis laws or have otherwise legalized the use of cannabis, the use of cannabis remains illegal under U.S. federal law for any purpose, by way of the Controlled Substances Act of 1970 (the "Controlled Substances Act").
The Company operates under one operating segment, which is the cultivation, production and sale of cannabis products.
The Company owns a portfolio of operating businesses, including:
The Issuer’s head office and registered office is located at 77 City Centre Drive, Suite 501 - East Tower, Mississauga, Ontario, L5B 1M5, Canada.
The Company’s telephone number is +1 (717) 610-4165 and its website is www.terrascend.com. Information contained on or accessible through the Company's website is not part of this Annual Report, and the inclusion of the Company's website address in this Annual Report is an inactive textual reference only.
Operating Businesses and Brands
The Company is a leading North American cannabis company. The Company has vertically integrated licensed operations in Pennsylvania, New Jersey, Michigan, Maryland and California. In addition, the Company has retail operations in Ontario, Canada with a majority-owned dispensary in Toronto, Ontario, Canada. The Company is committed to safely cultivating the highest quality cannabis products in order to elevate the lives of its patients and customers with a vision to keep growing and using the transformative power of cannabis to positively impact as many lives as possible. See the section titled “Acquisitions” for more information regarding the Company’s acquisitions.
The Company operates seven cultivation and processing facilities and thirty-eight operational dispensaries serving thousands of medical patients and adult-use consumers across North America. The Company offers six premium brands in a variety of product types including flower, vaporizables, concentrates, topicals, tinctures, and edibles. The Company also produces and sells an assorted product offering from two premium licensed brands, Cookies and Wana.
The Company’s in-house product brand portfolio has the following brand values:
7
The Company produces and sells products under third-party licensed brands in some markets, which consists of:
The Company's dispensary retail stores are branded as:
TerrAscend NJ
The Company, is a vertically integrated cultivator, processor and dispenser in New Jersey’s northern region. The Company, through its majority-owned subsidiary TerrAscend NJ, LLC, cultivates and processes medical and adult-use cannabis, and currently operates three Apothecarium-branded dispensaries in Phillipsburg, Lodi, and Maplewood, New Jersey. The Company operates a 16-acre site in Boonton Township, Morris County that currently has a cultivation and processing facility with a total footprint of approximately 140,000 square feet with the ability to further expand on the site. In addition to cultivation, the
8
Company is engaged in the manufacturing of a wide range of branded form factors including vaporizables, concentrates, topicals, tinctures and edibles.
The Company is the exclusive manufacturer, supplier, and commercialization partner of Wana and Cookies in New Jersey, producing these branded products for wholesale and retail distribution in the state.
TerrAscend MD
The Company is a vertically integrated cultivator, processor and dispenser in Maryland. The Company, through its wholly-owned subsidiary WDB Holding MD, Inc. (“WDB MD”) and TER Holding MD, Inc. (“TER MD”), holds one cultivation license, one processor license, and four retail licenses. The Company has a cultivation and processing operation, including a newly renovated state-of-the-art 150,000 square foot facility located in Hagerstown, Maryland. The Company is engaged in the extraction, processing and manufacturing of a wide-range of branded form factors including vaporizables, concentrates, topicals, tinctures and edibles. In addition, the Company operates four Apothecarium branded retail dispensaries, in Cumberland, Salisbury, Parkville, and Burtonsville.
The Company is the exclusive manufacturer, supplier, and commercialization partner of Wana and Cookies in Maryland, producing these branded products for wholesale and retail distribution in the state.
TerrAscend PA
The Company is a vertically integrated cultivator, processor and dispenser in Pennsylvania. The Company, through its wholly-owned subsidiary WDB Holding PA, Inc. (“WDB PA”), holds a cultivation, a processor, and six retail licenses in Pennsylvania. The Company, through Ilera Healthcare LLC (“Ilera”), has a 150,000 square foot grower and processor operation located in Waterfall, Pennsylvania. The Company distributes its product lines, including vaporizables, tinctures, and topicals broadly across dispensaries throughout Pennsylvania. In addition, the Company operates six Apothecarium branded retail dispensaries, in Plymouth Meeting, Lancaster, Thorndale, Bethlehem, Allentown and Stroudsburg. In addition to cultivation, the Company is engaged in the manufacturing of a wide range of branded form factors including vaporizables, concentrates, topicals, and tinctures.
The Company is the exclusive manufacturer, supplier, and commercialization partner of Cookies in Pennsylvania, producing these branded products for wholesale and retail distribution in the state.
TerrAscend MI
The Company is a vertically-integrated cultivator, processor and dispenser in Michigan. The Company, through its wholly-owned subsidiary WDB Holding MI, Inc. (“WDB MI”), holds nine operational “Class C” cultivation licenses, five Class C’s with local approvals, three excess grower licenses (equivalent to a Class C license), three processing licenses and nineteen provisioning centers or dispensaries. The Company has cultivation and processing facilities located in Harrison and Monitor, Michigan. The Company operates eight Gage branded dispensaries, five Pinnacle branded dispensaries, five Cookies branded dispensaries, and one Lemonnade branded dispensary.
The Company is the exclusive manufacturer, supplier, and commercialization partner of Cookies in Michigan, producing these branded products for wholesale and retail distribution in the state.
TerrAscend CA
The Company is a vertically-integrated cultivator and dispenser in California. The Company, through its wholly-owned subsidiary WDB Holding CA, Inc. (“WDB CA") holds licenses for cultivation, distribution, and retail operations. The Company has a cultivation operation located in San Francisco, California. The Company operates five Apothecarium branded retail dispensaries, including three in San Francisco, one in Berkeley and one in Capitola.
Florida Business - Arise
In 2023, TerrAscend, through its wholly-owned subsidiary Arise Bioscience, Inc. (“Arise”), engaged in the production and distribution of innovative hemp-derived wellness products. Arise’s whole-plant hemp extract products were made in the United States and were sold in retail locations in the United States. Effective March 1, 2023, the Company sold substantially all of the Arise assets, including all intellectual property and inventory, to a third party.
9
TerrAscend Canada
TerrAscend Canada sells cannabis in Ontario, Canada from its Cookies Canada retail dispensary. TerrAscend Canada’s principal business activities include the retail sale of recreational (“recreational” or “adult-use”) cannabis to consumers. TerrAscend Canada was previously a Licensed Producer (as such term is defined in the Cannabis Act, SC 2018, c 16 (the "Cannabis Act")) of cannabis until the Company commenced an optimization of its operations in Canada, whereby the Company reduced its manufacturing footprint to focus on its Canadian retail business, as well as to monetize its intellectual property portfolio in Canada. Prior to the optimization of its operations, TerrAscend Canada operated out of a 67,300 square foot facility located in Mississauga, Ontario and was licensed to cultivate, process and sell cannabis for medical and adult-use purposes. These licenses allowed for sales of dried cannabis, cannabis oil and extracts, topicals, and edibles. The Company ceased operations at TerrAscend Canada’s manufacturing facility during the three months ended December 31, 2022. As such, the Canadian Licensed Producer results are presented in discontinued operations in this Annual Report.
Reorganization
Entry into the U.S. Cannabis Market and Capital Reorganization
The Company was incorporated under the Business Corporations Act (Ontario) on March 7, 2017. At the time the Company had limited operations in the United States and did not engage in the business of, or derive any revenue from, the cultivation, distribution or possession of cannabis in the United States. On October 9, 2018, the Company announced its intention to pursue growth opportunities in the U.S. cannabis market, including potential acquisitions of operators in states that had legalized cannabis for medical or adult-use. In connection therewith, the Company began exploring potential acquisition targets with significant market share and strong brand recognition. To support this strategy, the Company entered into an agreement with Canopy Growth Corporation (“Canopy Growth”), RIV Capital Inc. (formerly Canopy Rivers Inc., “RIV Capital”), and entities controlled by Jason Wild, chairman of the Company (JW Opportunities Master Fund, Ltd., JW Partners, LP, and Pharmaceutical Opportunities Fund, LP) to reorganize the capital of the Company (the "Reorganization”) and obtain waivers of certain contractual covenants that at the time, restricted the Company from operating in the United States. The Company's Reorganization was implemented by way of a statutory plan of arrangement on the terms set out in the Arrangement Agreement and was subject to court approval, the approval of the Company’s shareholders, and other customary conditions. The Reorganization was completed on November 30, 2018.
Acquisitions
Acquisition of Grander Assets
On December 24, 2018, the Company announced the signing of a definitive agreement to acquire substantially all of the assets of Grander, a manufacturer and distributor of hemp-derived wellness products. The transaction closed on January 15, 2019. Substantially all of the operating assets of Grander were indirectly acquired by the Company through Arise, a wholly-owned subsidiary of TerrAscend. Grander ceased operations in 2024.
Acquisition of the Apothecarium Dispensaries in California
On June 6, 2019, the Company closed a series of transactions to acquire controlling interests in three entities in California operating under The Apothecarium-branded retail dispensary ("Apothecarium 2019 Acquisition"). The transactions also included the acquisition of two additional entities that were seeking to operate in Northern California, which were ultimately opened in Berkeley and Capitola, and the acquisition of Valhalla, a leading provider of premium edible products. The Company acquired 49.9% of the outstanding equity interests of the entities operating three Apothecarium-branded retail dispensary located in San Francisco and had the right (or, in certain circumstances, obligation) to acquire the remaining equity interests of such entities post-closing, following receipt of certain regulatory approvals. As consideration, the Company paid $71,854, comprised of: (i) $36,837 in cash, (ii) $813 in the form of a working capital adjustment, (iii) contingent consideration of $3,363 and (iv) 6,700 proportionate voting shares in the capital of the Company (“Proportionate Voting Shares”), valued at $30,841. TerrAscend retained 100% of the economics of the entities operating the three San Francisco locations and the Berkeley and Capitola locations of The Apothecarium dispensaries through control of such entities. On January 19, 2024, the Company acquired the remaining 50.1% of the outstanding equity interest in the three the Apothecarium-branded retail dispensary located in San Francisco, each, therefore, becoming a whole-owned subsidiary of TerrAscend ("Apothecarium 2024 Acquisition"). As consideration, the Company paid $3,700, comprised of 2,105,549 Common Shares.
10
Acquisition of Ilera
On September 16, 2019, the Company, through WDB Holding PA, a wholly-owned subsidiary of TerrAscend, acquired 100% of the equity of the entities comprising Ilera for total consideration of $225,000 comprised of a combination of cash and Common Shares. At closing, the Company paid the sellers $25,000 in cash, subject to customary closing adjustments, an additional $25,000 worth of Proportionate Voting Shares equivalent to approximately 5,059.102 Proportionate Voting Shares (which are each exchangeable for 1,000 Common Shares), and $601,000 in working capital adjustments. Additional cash consideration of $175,000 in aggregate was paid to the sellers based on Ilera achieving certain specified sales and profitability targets, with staggered payments made in 2020 and 2021. On June 30, 2021 the final earn-out had been calculated and remaining fair value amount of $29,700 was paid on that date.
Acquisition of State Flower
On January 23, 2020, the Company obtained control of ABI SF, LLC ("State Flower"), a premium California cannabis brand that is currently sold through dispensaries in California. As consideration, the Company converted its previously-issued note receivable and accrued interest in the amount of $3,032 into a 49.9% equity interest in State Flower ("State Flower Acquisition"). The Company also recorded contingent consideration payable of $6,630, representing the expected consideration payable to acquire the remaining 50.1% of State Flower, which comprises 100% of its preferred shares, subject to regulatory approval. The Company retained 100% of the economics of State Flower through control of State Flower and has the right to acquire the remaining equity interests of State Flower, following receipt of certain regulatory approvals. On December 31, 2021, the final earn-out was calculated. The Company made a payment of $7,000 in January 2022. On January 19, 2024, the Company acquired the remaining 50.1% of the equity interest in State Flower, as a result of which State Flower became a wholly-owned subsidiary of TerrAscend.
Acquisition of HMS
On May 3, 2021, the Company, through WDB MD, a wholly-owned subsidiary of TerrAscend, acquired HMS Health, LLC ("HMS Health"), a cultivator of cannabis flower for the wholesale medical cannabis market in Maryland. The Company acquired 100% of the equity of HMS Health as well as the rights to acquire 100% of the equity interest in HMS Processing, LLC ("HMS Processing"), a processor of cannabis flower, following receipt of certain regulatory approvals, for total consideration of $24,488, comprised of: (i) $22,399 in cash and (ii) a $2,089 promissory note, bearing 5.0% annual interest, which was settled when due in April 2022. On January 31, 2022, the Company acquired 100% of the interest in HMS Processing, which resulted in both HMS Processing and HMS Health becoming wholly-owned subsidiaries of TerrAscend.
Acquisition of KCR
On April 30, 2021, the Company, through WDB Holding PA, a wholly-owned subsidiary of TerrAscend, acquired Guadco, LLC and KCR Holdings LLC (collectively “KCR”). In connection with the acquisition of KCR ("KCR Acquisition"), the Company acquired three retail dispensaries located in Bethlehem, Allentown and Stroudsburg, Pennsylvania. Prior to the acquisition, the Company owned 10% of KCR. The Company acquired the remaining 90% of the equity interest in KCR for total consideration of $69,847, comprised of: (i) $34,427 in Common Shares, (ii) $20,506 in cash, (iii) $7,101 related to the fair value of previously owned shares, (iv) a contingent consideration payable, with a value of $1,063 , and (v) a $6,750 promissory note, bearing 10% annual interest, which was settled when due in April 2022.
New Jersey Partnership
On August 20, 2021, the Company purchased an additional 12.5% equity interest in TerrAscend NJ, LLC from BWH NJ, LLC and Blue Marble Ventures, LLC (in addition to its existing 75% equity interest). The total cash consideration was $50,000, which was paid during the year ended December 31, 2021. Upon closing of the acquisition, the Company owns 87.5% of the issued and outstanding equity of TerrAscend NJ, LLC.
The Company had the option to purchase an additional 6.25% equity interest in TerrAscend NJ, LLC, for a total equity interest of 93.75%, at a predetermined valuation during the period commencing April 1, 2023 through June 15, 2023, but did not elect to exercise.
Acquisition of Gage Growth
On March 10, 2022, the Company closed the acquisition of Gage Growth (the "Gage Acquisition"), pursuant to terms of an arrangement agreement between the Company and Gage Growth (the "Gage Arrangement Agreement"). Pursuant to the terms of the Gage Arrangement Agreement, the Company acquired 100% of the issued and outstanding subordinate voting shares of Gage Growth. Pursuant to the terms of the Gage Arrangement Agreement, Gage shareholders received .3001 of a Common
11
Share for each Gage share (or equivalent) held. In connection with the Gage Acquisition, the Company issued: (i) 51,349,978 Common Shares valued at $242,884, (ii) 13,504,500 exchangeable share units valued at $66,591, (iii) 4,940,364 replacement stock options with a fair value of $13,147, and (iv) 282,023 replacement warrants with a fair value of $435.
Acquisition of Pinnacle
On August 23, 2022, the Company acquired 100% of the outstanding equity interests in Pinnacle, a dispensary operator in Michigan, and related real estate, for total consideration of $31,003, comprised of: (i) $12,327 in cash (the "Pinnacle Cash Consideration"), (ii) two promissory notes in an aggregate amount of $10,000, each bearing interest at a rate of 6.0% which was to mature on June 23, 2023, (iii) 4,803,184 Common Shares valued at $7,926 (the "Pinnacle Share Consideration"), and (iv) contingent consideration payable, valued at $750. Subject to compliance with securities laws, the Common Shares issued in connection with the Pinnacle Share Consideration were subject to a contractual lock-up period, with one-third of the Common Shares vesting on each of the 30, 60 and 90 day anniversary of the closing date of the transaction. The Pinnacle Cash Consideration included repayments of indebtedness and transaction expenses on behalf of Pinnacle of $3,913 and $619 respectively. In connection with the Pinnacle Acquisition, the Company acquired six retail dispensary licenses, five of which are currently operational and located in the cities of Addison, Buchanan, Camden, Edmore, and Morenci, Michigan.
Acquisition of AMMD
On January 8, 2022, the Company entered into a definitive agreement to acquire Allegany Medical Marijuana Dispensary ("AMMD"), a medical dispensary located in Cumberland, Maryland from Moose Curve Holdings, LLC (the "AMMD Acquisition"). On January 27, 2023, the Company closed the AMMD Acquisition. Pursuant to the terms of the agreement, the Company acquired a 100% of the outstanding equity interest in AMMD for total consideration of $10,000 in cash (the "AMMD Cash Consideration). The AMMD Cash Consideration paid included a long-term lease with the option to purchase the real estate and repayments of indebtedness and transaction expenses on behalf of AMMD of $160 and $29, respectively.
Acquisition of Peninsula
On June 28, 2023, in order to expand its retail footprint in Maryland, the Company closed the acquisition of Derby 1, LLC ("Peninsula"), a dispensary located in Salisbury, Maryland (the "Peninsula Acquisition"). Pursuant to the terms of the agreement, the Company acquired 100% of the outstanding equity interests in Peninsula for total consideration of $15,394 exclusive of assumed financing obligations of $7,226. The consideration was comprised of: (i) 5,442,282 Common Shares (the "Peninsula Share Consideration"), valued at $7,857, (ii) a $3,646 secured promissory note bearing interest at a rate of 7.25% and maturing on June 28, 2026, and (iii) $2,657 of contingent consideration in connection with the Peninsula Share Consideration ("Peninsula Contingent Consideration"), and (iv) $1,234 in cash (the "Peninsula Cash Consideration"). The Peninsula Cash Consideration included transaction expenses and repayments of indebtedness on behalf of Peninsula of $290 and $33, respectively. The Peninsula Share Consideration was subject to a statutory lock-up restriction of 6 months, and therefore, a share restriction discount was considered in determining the fair value of the Peninsula Share Consideration on the date of issuance. Pursuant to the terms of the agreement, the Company agreed that if within 18 months from the date of issuance of the Peninsula Share Consideration, the aggregate gross proceeds resulting from the sales of the Common Shares plus the aggregate value of the remaining Common Shares was less than $9,000, the Company was required to pay the difference to the sellers.
Acquisition of Blue Ridge
On June 30, 2023, the Company closed the acquisition of Hempaid, LLC ("Blue Ridge"), a medical dispensary located in Parkville, Maryland (the "Blue Ridge Acquisition"). Pursuant to the terms of the agreement, the Company acquired a 100% equity interest in Blue Ridge for total consideration of $6,277, comprised of: (i) a promissory note of $3,109 bearing interest at a rate of 7.0% and maturing on June 30, 2027 and (ii) $3,168 in cash (the "Blue Ridge Cash Consideration"). The Blue Ridge Cash Consideration included repayments of indebtedness and transaction expenses on behalf of Blue Ridge of $707 and $281, respectively. The Company has plans to relocate Blue Ridge to a new, high-traffic retail center in the beginning of 2024.
Acquisition of Herbiculture
On July 10, 2023, the Company closed the acquisition of Herbiculture Inc. (“Herbiculture”), a medical dispensary in Maryland (the "Herbiculture Acquisition"). Pursuant to the terms of the agreement, the Company acquired 100% equity interest in Herbiculture for total consideration of $7,710, comprised of: (i) $2,776 in cash ("Herbiculture Cash Consideration"), and (ii) a promissory note of $4,934 bearing interest at a rate of 10.50%, and maturing on June 30, 2026. The Herbiculture Cash Consideration included transaction expenses and repayments of indebtedness on behalf of Herbiculture of $616 and $1,674, respectively.
12
Principal Products
The Company offers a competitive product portfolio, ranging from flower, concentrates, vaporizables, edibles, tinctures, topicals, and/or accessories, in the jurisdictions in which it operates. The Company strives to develop and introduce innovative products to serve patients’ and customers’ unique needs.
Principal Markets
The Company currently has operations in Canada, through TerrAscend Canada, and in the United States, through TerrAscend. In the United States, TerrAscend sells cannabis products in Michigan, Pennsylvania, New Jersey, Maryland and California. In Canada, TerrAscend Canada operates its Cookies Canada retail business and is monetizing its intellectual property portfolio. The Company’s business is not generally cyclical or seasonal in nature.
Distribution Methods
In the United States, TerrAscend distributes its branded products across dispensaries in Pennsylvania, New Jersey, Maryland, Michigan and California. In Canada, the Company sells its products to consumers in Ontario through TerrAscend Canada.
Sources and Availability of Materials
The Company either grows or procures the primary component of its finished products, namely cannabis. TerrAscend’s cultivation operations in the United States are dependent on a number of key inputs and their related costs including raw materials and supplies related to its growing operations, as well as electricity, water and other utilities.
Specialized Knowledge and Skills
The Company’s business requires specialized skills and knowledge. The Company believes its team has developed and sourced business systems to effectively and efficiently operate its wholesale operations and retail cannabis operations in the jurisdictions in which it operates in the United States and Canada. The Company believes that the brand building, retail marketing and product development knowledge and skills of the Company’s management team and employees possess will be essential to the Company becoming a respected household name within the retail cannabis industry. Please see Item 1A – “Risk Factors” – “Risks Related to the Company’s Business, Operations and Industry” – “The Company is dependent on suppliers and key inputs for the cultivation, extraction and production of cannabis products.”
Competitive Conditions
The Company currently faces, and will continue to face, competition from new and existing licensed cannabis operators, competitors with existing retail operations, government owned retailers, the illicit market, and other applicable participants in the cannabis wholesale and manufacturing industry. Some of the Company's competitors may have greater financial resources, market access and manufacturing and marketing experience than the Company. Due to challenging market conditions in certain jurisdictions, some operators have exited the industry and in doing so have heavily discounted their products, creating pressure on pricing.
Increased competition from numerous independent cannabis retail outlets, wholesalers and larger and better-financed competitors (including new entrants), and heavily discounted products by exiting players could have a material adverse effect on the Company. In the United States, as each state continues to oversee all regulations and policies within its individual borders, each state has the power to change its guidelines, laws, and regulatory operations at any time. This includes, but is not limited to expanding into adult-use consumption, limiting the number of dispensaries an operator can own and run in a jurisdiction, or regulating partnerships with social equity licenses.
The Company’s competitors can be grouped into the following categories:
13
Protection of Intellectual Property
The Company believes that the ownership and protection of the Company’s intellectual property rights is a significant aspect of the Company’s future opportunity. Currently the Company relies on trade secrets, technical know-how and proprietary information for its commercial strategy. The Company protects its intellectual property by seeking and obtaining registered protection where possible, developing and implementing standard operating procedures to protect trade secrets, technical know-how and proprietary information and entering into restrictive agreements with parties that have access to the Company’s inventions, trade secrets, technical know-how and proprietary information, such as the Company’s partners, collaborators, employees and consultants, to protect confidentiality and intellectual property rights. The Company also seeks to preserve the integrity and confidentiality of its inventions, trade secrets, trademarks, technical know-how and proprietary information by maintaining strict operating procedures, physical security of the Company’s premises and physical and electronic security of its information technology systems.
In addition, the Company has sought and will continue to seek trademark protection in the United States and Canada. The Company’s ability to obtain registered trademark protection for cannabis-related goods and services, in particular for cannabis itself is limited in the United States, where registered federal trademark protection is currently unavailable for trademarks covering cannabis-related products and services that are illegal under the Controlled Substances Act. Accordingly, the Company’s ability to obtain intellectual property rights or enforce intellectual property rights against third party uses of similar trademarks may be limited in the United States. The U.S. Patent and Trademark Office released a policy on May 2, 2019 which clarifies that applications for trademarks for products that meet the definition of hemp could be accepted for registration, subject to certain exceptions.
Privacy and Cybersecurity
In the ordinary course of our business, the Company may process personal or sensitive data. Accordingly, the Company is subject to numerous data privacy and security obligations, including federal, state, local, and foreign laws, regulations, guidance, and industry standards related to data privacy, security, and protection. Such obligations may include, without limitation, the Federal Trade Commission Act, the Telephone Consumer Protection Act of 1991 (“TCPA”), the Controlling the Assault of Non-Solicited Pornography and Marketing Act of 2003, the California Consumer Privacy Act of 2018 (“CCPA”), the Canadian Personal Information Protection and Electronic Documents Act, Canada’s Anti-Spam Legislation, and the Payment Card Industry Data Security Standard (“PCI-DSS”). Additionally, the Company is, or may become, subject to various U.S. federal and state consumer protection laws which require us to publish statements that accurately and fairly describe how the Company handles personal data and choices individuals may have about the way their personal data was handled.
The regulatory landscape around privacy and cybersecurity is evolving rapidly and becoming increasingly stringent, which may increase our compliance obligations and exposure for any noncompliance. See the section titled “The Company is or may become subject to stringent and evolving U.S. and foreign laws, regulations, rules, contractual obligations, policies and other obligations related to data privacy and security. Its actual or perceived failure to comply with such obligations could lead to regulatory investigations or actions; litigation; fines and penalties; disruptions of the Company's business operations; reputational harm; loss of revenue or profits; and other adverse business consequences” for additional information about the laws and regulations to which we may become subject and about the risks to our business associated with such laws and regulations.
14
Human Capital
As of December 31, 2023, the Company employed 1,165 employees, 73 of whom were subject to a collective bargaining agreement. Of these employees, approximately 944 were engaged on a full-time basis. The Company believes that it has a good relationship with its employees.
The Company believes in building a diverse team, and it strives to foster a welcoming culture where employees can make an impact on the Company’s success. The Company encourages talented people from all backgrounds to join the Company and dedicated to promoting inclusion by encouraging its employees to participate in employee resource groups and other diversity, equity, and inclusion initiatives.
The Company is committed to providing a safe and secure work environment in accordance with applicable labor, safety, health, anti-discrimination and other workplace laws. The Company strives for all employees to feel safe and empowered at work. To that end, The Company maintains a hotline that employees can call, with the option of remaining anonymous, to voice concerns.
Licenses and Regulatory Framework in United States
Summary of U.S. Cannabis Regulatory Regime
The cannabis industry is subject to various state and local laws, regulations and guidelines relating to the cultivation, manufacture, distribution, sale, storage and disposal of medical and adult-use cannabis, as well as laws and regulations relating to health and safety, the conduct of operations and the protection of the environment. The U.S. regulatory scheme varies in its terminology and definitions, using “cannabis”, “marijuana,” “marihuana,” and “hemp” as distinct terms. The regulatory environment governing the medical and adult-use cannabis industries in the United States, where state law permits such activities, are, and will continue to be, subject to evolving regulation by governmental authorities. Accordingly, there are a number of risks associated with investing in businesses in an evolving regulatory environment, including, without limitation, increased number of permits or licenses being issued and increased regulatory burden on operators.
The U.S. federal government classifies cannabis as a Schedule I controlled substance under the Controlled Substances Act. Cannabis and/or cannabis products having more than 0.3% concentration of delta-9 tetrahydrocannabinol, or THC, is cannabis. Conversely, cannabis with a THC concentration of less than 0.3% is classified as hemp.
There are 38 states plus the District of Columbia, the Commonwealth of the Northern Mariana Islands, Puerto Rico, United States, Virgin Islands and Guam have legalized medical cannabis and approximately 24 states plus the District of Columbia, Guam, and the Commonwealth of Northern Marina Islands have legalized adult-use cannabis. Notwithstanding the permissive regulatory environment of medical, and in some cases also adult-use cannabis at the state level, cannabis remains a Schedule I drug under the Controlled Substances Act, making it illegal under U.S. federal law to cultivate, manufacture, distribute, sell or possess cannabis in the United States. Furthermore, financial transactions involving proceeds generated by, or intended to promote, cannabis-related business activities in the United States may form the basis for prosecution under applicable federal money laundering legislation.
The U.S. federal government’s approach to enforcement of cannabis laws has trended toward deferring to state laws where a robust state regulatory framework exists. On August 29, 2013, the U.S. Department of Justice (the “DOJ”) issued a memorandum known as the “Cole Memorandum” to all U.S. Attorneys’ offices. The Cole Memorandum generally directed U.S. Attorneys not to prioritize the enforcement of federal cannabis laws against individuals and businesses that comply with state medical cannabis programs. The Cole Memorandum, while not legally binding and only a policy statement, assisted in managing the tension between state and federal laws concerning all medical and adult-use state-regulated cannabis businesses.
On January 4, 2018, the Cole Memorandum was rescinded by former U.S. Attorney General Jeff Sessions. While this did not create a change in federal law, the revocation added to the uncertainty of U.S. federal enforcement of the Controlled Substances Act in states where cannabis use is regulated. Former Attorney General Sessions also issued a one-page memorandum known as the “Sessions Memorandum” which confirmed the rescission of the Cole Memorandum and explained that the Cole Memorandum was “unnecessary” due to existing general enforcement guidance as set forth in the U.S. Attorney’s Manual. The Sessions Memorandum did not otherwise indicate that the prosecution of cannabis-related offenses is a heightened DOJ priority. Furthermore, the Sessions Memorandum explicitly describes itself as a guide to prosecutorial discretion. Such prosecutorial discretion remains in the hands of U.S. Attorneys when deciding whether or not to prosecute cannabis-related offenses.
15
On November 7, 2018, U.S. Attorney General Sessions resigned as U.S. Attorney General. On February 14, 2019, William Barr was confirmed by the U.S. Senate (the "Senate") as the next U.S. Attorney General. During one of his Senate confirmation hearings, Mr. Barr stated that he did not support cannabis legalization but would not prosecute cannabis businesses that comply with state laws. Mr. Barr stated further that he would not upset settled expectations that have arisen as a result of the Cole Memorandum.
On March 11, 2021, Merrick Garland was appointed U.S. Attorney General and indicated he would generally act in accordance with the Cole Memorandum, when, at his confirmation hearing, he said, “It does not seem to me a useful use of limited resources that we have, to be pursuing prosecutions in states that have legalized and that are regulating the use of cannabis, either medically or otherwise.” U.S. Attorney General. Mr. Garland has not, however, reissued the Cole Memorandum or issued substitute guidance.
On October 6, 2022, President Joseph Biden requested that the Secretary of the U.S. Department of Health and Human Services (“HHS”) and the U.S. Attorney General initiate a review as to how cannabis is currently scheduled under federal law. In December 2022, President Biden signed the Medical Marijuana and Cannabidiol Research Expansion Act into law, which allows for significantly broader opportunities to study cannabis. On August 29, 2023, following a review by the U.S. Food and Drug Administration (“FDA”), the Secretary of HHS issued a recommendation to the Drug Enforcement Administration (“DEA”) that cannabis be moved from Schedule I to Schedule III under the Controlled Substances Act. In December 2023, the DEA confirmed it is in the process of conducting its review of HHS’s recommendation.
Despite it’s recension, as an industry best practice, the Company abides by the following policies to ensure compliance with the guidance included in the Cole Memorandum:
The Company’s operations comply with all licensing requirements as required by the state, county, municipality, or other administrative divisions and the Company continuously monitors its operations to ensure compliance of such operations;
The Company has implemented compliant policies and procedures to prevent the distribution of cannabis products to minors;
The Company has implemented an inventory tracking system and other compliant procedures to track inventory and prevent the diversion of cannabis products in compliance with each jurisdiction’s requirements;
The Company’s operations adhere to the scope of the regulations governing the cannabis license in the market in which the Company operates;
The Company has implemented compliant policies and procedures to prevent the distribution of the proceeds from its operations to criminal enterprises, cartels, or gangs;
The Company has implemented compliant quality controls to ensure all products comply with applicable regulations and contain the necessary disclaimers and warnings associated with the contents of the products to avoid adverse public health consequences; and
The Company has implemented compliant policies and procedures to effectively monitor its state-authorized operations so that it is not used as a cover or pretense for the trafficking of illegal drugs or engaging in other illegal activity.
For the reasons set forth herein, the Company’s existing investments in the United States, and any future investments, may become the subject of heightened scrutiny by regulators, stock exchanges and other authorities in the United States or Canada. As a result, the Company may be subject to significant direct and indirect interaction with public officials. There can be no assurance that this heightened scrutiny will not lead to the imposition of certain restrictions on the Company’s ability to invest in the United States or any other jurisdiction. Government policy changes or public opinion may also result in a significant influence over the regulation of the cannabis industry in the United States or elsewhere. Among other things, such a shift could cause state and local jurisdictions to abandon initiatives or proposals to legalize medical or adult-use cannabis, thereby limiting the number of new state jurisdictions into which the Company could expand. Any inability to fully implement the Company’s expansion strategy may have a material adverse effect on the Company’s business, financial condition and results of operations.
Additionally, under U.S. federal law, it may be a violation for financial institutions to take any proceeds from the sale of cannabis or any other Schedule I controlled substance. Banks and other financial institutions, particularly those that are federally chartered in the United States, could be prosecuted and possibly convicted of money laundering for providing services to
16
cannabis businesses. It may also be a violation of federal money laundering statutes for “federal health care law violations,” which include violations of the Federal Food, Drug, and Cosmetic Act (“FDCA”).
Violations of any U.S. federal laws and regulations could result in significant fines, penalties, administrative sanctions, convictions or settlements arising from civil proceedings conducted by either the federal government or private citizens, or criminal charges, including, but not limited to, disgorgement of profits, cessation of business activities, civil forfeiture or divestiture. This could have a material adverse effect on the Company, including its reputation and ability to conduct business, its cannabis licenses in the United States, the listing of its securities on various stock exchanges, its financial position, operating results, profitability or liquidity or the market price of its publicly traded shares. In addition, it is difficult for the Company to estimate the time or resources that would be needed for the investigation of any such matters or its final resolution because, in part, the time and resources that may be needed are dependent on the nature and extent of any information requested by the applicable authorities involved, and such time or resources could be substantial. For the reasons set forth above, the Company’s investments and operations in the United States may become the subject of heightened scrutiny by regulators, stock exchanges and other authorities in Canada.
The Company may also be subject to a variety of laws and regulations domestically and in the United States that relate to money laundering, financial recordkeeping and proceeds of crime, including the Currency and Foreign Transactions Reporting Act of 1970 (commonly known as the "Bank Secrecy Act"), as amended by Title III of the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001 (USA PATRIOT Act), the Proceeds of Crime (Money Laundering) and Terrorist Financing Act (Canada), as amended and the rules and regulations thereunder, the Criminal Code (Canada) and any related or similar rules, regulations or guidelines, issued, administered or enforced by governmental authorities in the United States and Canada.
In February 2014, the Financial Crimes Enforcement Network of the Treasury Department issued a memorandum (the “FinCEN Memorandum”) providing instructions to banks seeking to provide services to cannabis-related businesses. The FinCEN Memorandum clarifies how financial institutions can provide services to cannabis-related businesses consistent with their Bank Secrecy Act obligations. It refers to supplementary guidance that Deputy Attorney General Cole issued to federal prosecutors relating to the prosecution of money laundering offenses predicated on cannabis-related violations of the Controlled Substances Act and independently lists the federal government’s enforcement priorities as related to cannabis. Although the original FinCEN Memorandum is still in place, this supplementary DOJ guidance that accompanied the FinCEN Memorandum was rescinded when former Attorney General Sessions rescinded the Cole Memorandum. It is unclear whether the current administration will follow the guidelines of the FinCEN Memorandum, although immediately after the Sessions Memorandum, then-U.S. Treasury Secretary Steven Mnuchin stated that the Treasury Department had no intention to rescind the FinCEN Memorandum but, instead, wanted to improve the availability of banking services in the state-regulated cannabis space.
H.R. 1595, or "the SAFE Banking Act" of 2019, which would expand financial services in the United States. to cannabis-related legitimate businesses and service providers, was introduced in the U.S. House of Representatives (the “House”) on March 7, 2019 with bipartisan support. The Safe Banking Act has passed the House six times but has yet to pass the Senate. A new version of the SAFE Banking Act known as the Secure and Fair Enforcement Regulation ("SAFER Banking Act") was introduced in the Senate on September 21, 2023, and subsequently approved by the Senate Committee on Banking. The SAFER Banking Act is now pending passage in the U.S. Senate and, if passed, will move on to the House where it faces an uncertain future.
Other legislation that has been introduced in the United States that would make cannabis transactions easier and more predictable, include the Marijuana Opportunity Reinvestment and Expungement Act (the “MORE Act”) and the Cannabis Administration and Opportunities Act (the “CAOA”). The MORE Act was first introduced in July 2019 by Representative Jerrold Nadler in the House, and in the Senate by then-U.S. Senator Kamala Harris. If it were to become law, the MORE Act would remove cannabis as a Schedule I controlled substance under the Controlled Substances Act and make available U.S. Small Business Administration funding for regulated cannabis operators. The MORE Act was reintroduced in the current U.S. Congress (“Congress”) by Representative Nadler on May 28, 2021, with no corresponding bill introduced in the Senate. The CAOA was released as a discussion draft by U.S. Senate Majority Leader Chuck Schumer, U.S. Senator Ron Wyden, and U.S. Senator Cory Booker in July 2021. If it were to become law it would, among other things, remove cannabis from the definition of a controlled substance under the Controlled Substances Act, allow states to set their own regulations for cannabis, and block states from prohibiting interstate commerce of regulated cannabis across their borders. Recently introduced into Congress is another bill, the States Reform Act, introduced by Rep. Nancy Mace (R-SC), which would repeal the federal prohibition of cannabis.
Despite the rescission of the Cole Memorandum, Congress has passed appropriations bills in each fiscal year since FY2015, that prevents the federal government from using congressionally appropriated funds to enforce federal cannabis laws against regulated medical cannabis actors operating in compliance with state and local law. The continuing resolution contains, among
17
other things, a rider known as Rohrabacher-Blumenauer Amendment (the "RBA"), which prevents the federal government from using congressionally appropriated funds to enforce federal cannabis laws against regulated medical cannabis actors operating in compliance with state medical laws. However, this measure does not protect adult-use cannabis businesses. The U.S. Ninth Circuit in United States v. McIntosh held that the prohibition under the RBA also prevents the DOJ from spending federal funds to prosecute individuals who are engaged in conduct that is permitted by, and in compliance with, state medical cannabis laws.
On October 6, 2022, President Joseph Biden requested that the Secretary of HHS and the Attorney General to initiate a review as to how cannabis is currently scheduled under federal law. In August 2023, following a review by the FDA, the Secretary of HHS issued a recommendation to the DEA that cannabis be moved to Schedule III under the Controlled Substances Act. In December 2023, the DEA confirmed it is currently conducting its review.
State-Level Overview
The following section presents an overview of market and regulatory conditions for the cannabis industry in the United States that the Company has or is intending to have an operating presence and is presented as of the date of filing, unless otherwise indicated.
California
In 1996, California voters passed Proposition 215, also known as the Compassionate Use Act, allowing physicians to recommend cannabis for an inclusive set of qualifying medical conditions. The law established a not-for-profit patient/caregiver system but there was no state licensing authority to oversee the businesses that emerged. In September of 2015, the California legislature passed three bills, collectively known as the Medical Marijuana Regulation and Safety Act. In 2016, California voters passed The Adult Use of Marijuana Act, which legalized recreational use cannabis for adults 21 years of age and older and created a licensing system for commercial cannabis businesses. On June 27, 2017, then-Governor Jerry Brown signed Senate Bill 94 into law, which combined California’s medicinal and recreational use cannabis frameworks into one licensing structure under the Medicinal and Adult-Use of Cannabis Regulation and Safety Act (“MAUCRSA”).
Pursuant to MAUCRSA: (i) CalCannabis, a division of the California Department of Food and Agriculture, was designated to issue licenses to cannabis cultivators: (ii) the Manufactured Cannabis Safety Branch (the “MCSB”), a division of the California Department of Public Health, was designated to issue licenses to cannabis manufacturers; and (iii) the California Department of Consumer Affairs, via its agency the Bureau of Cannabis Control (the “BCC”), was designated to issue licenses to cannabis distributors, testing laboratories, retailers, and micro-businesses. These agencies were also charged with overseeing various aspects of implementing and maintaining California’s cannabis landscape, including the statewide track and trace system. All three agencies released their initial emergency rulemakings at the end of 2017 and updated them with minor revisions in June 2018. The three agencies adopted their permanent rulemakings on January 16, 2019. All three agencies began issuing temporary licenses in January 2018 and stopped doing so on December 31, 2018, pursuant to MAUCRSA.
Local authorization is a prerequisite to obtaining a state license, and local governments are permitted to prohibit or otherwise regulate the types and number of cannabis businesses allowed in their locality. All three state regulatory agencies require confirmation from the applicable locality that the operator is operating in compliance with local requirements and was granted authorization to continue or commence commercial cannabis operations within the locality’s jurisdiction. Applicants are required to comply with all local zoning and land use requirements and provide written authorization from the property owner where the commercial cannabis operations are proposed to take place, which must dictate that the applicant has the property owner’s authorization to engage in the specific state-sanctioned commercial cannabis activities proposed to occur on the premises. The State has not set a limit on the number of state licenses an entity may hold, unlike other states that have restricted how many cannabis licenses an entity may hold in total or for various types of cannabis activity. Although vertical integration across multiple license types is allowed under MAUCRSA, testing laboratory licensees may not hold any other licenses aside from a laboratory license. There are also no residency requirements for ownership of a state license under MAUCRSA.
California state licenses, and some local licenses, are renewed annually. Each year, licensees are required to submit a state renewal application to the relevant regulatory authority, and all applicable local renewal applications to the applicable local regulatory body (for local licenses).
On July 12, 2021, Governor Gavin Newsom signed AB-141 into law, triggering the consolidation of CalCannabis, the MCSB, and the BCC into the newly created Department of Cannabis Control (the “DCC”). The DCC was created in an effort to centralize regulatory authority and facilitate a more easily navigable regulatory regime. All licenses obtained under the previous regulatory authorities automatically transferred to the DCC, which is now responsible for issuing and renewing all cannabis licenses. In September 2021 the DCC issued emergency regulations, which were approved and went into effect the same month.
18
The emergency regulations, among other things, include revised definitions clarifying who are considered to be owners or holders of a financial stake in cannabis businesses, and provisions allowing for the sale of branded products between businesses.
California’s robust regulatory system is designed to ensure, monitor, and enforce compliance with all aspects of a cannabis operator’s licensed operations. California’s state license application process additionally requires comprehensive criminal, regulatory, financial and personal disclosures, coupled with stringent monitoring and continuous reporting requirements designed to ensure only good actors are granted licenses and that licensees continue to operate in compliance with the state regulatory program. Applicants must submit standard operating procedures describing how the operator will, among other requirements, secure the facility, manage inventory, comply with the state’s seed-to-sale tracking requirements, dispense cannabis, and handle waste, as applicable to the license sought. Once licensed, an operator must continue to abide by the processes described in its application and seek regulatory approval before any changes to such procedures can be made. Licensees are additionally required to train their employees on compliant operations and are only permitted to transact with other legal and licensed businesses.
As a condition of state licensure, operators must consent to random and unannounced inspections of their commercial cannabis facility as well as the facility’s books and records, so as to monitor and enforce compliance with state law. Many localities have also enacted similar standards for inspections, and the state has already commenced site-visits and compliance inspections for operators who have received state temporary or annual licensure.
New Jersey
On January 18, 2010, the Compassionate Use Medical Marijuana Act (the “CUMMA”) came into force allowing patients with a limited number of qualifying medial conditions to access the state’s medical marijuana program. The New Jersey Department of Health (the “NJDOH”) issued regulations shortly thereafter authorizing the NJDOH to accept applications for a minimum of six alternative treatment centers (the “ATCs”), with two each to operate in the north, central and south regions of New Jersey.
CUMMA permits each ATC to operate as both a cultivator and dispensary under one permit. These activities can take place at up to two locations, as long as both locations are within the same region. The application process involves two stages. Those seeking an ATC permit must first submit an application seeking authority to apply for a permit to operate. Upon the granting of the application, the prospective ATC must then complete the application for actual permitting. Applications for authority to apply for a permit may only be submitted following solicitation from NJDOH for such applications. The first six permits for ATCs were awarded to nonprofit entities, with subsequent permits to be available to both nonprofit and for-profit entities. In 2013, CUMMA was amended to allow ATCs to cultivate an unlimited number of strains of cannabis and sell additional cannabis-infused products, and to restrict the same of cannabis-infused edible products to qualifying patients under the age of 18. With additional authorizations, ATCs may also house manufacturing facilities for cannabis-infused products such as syrups and lozenges. All cannabis is subject to a tetrahydrocannabinol (“THC”) limit of 10%, though NJDOH is proposing to repeal the regulation that establishes this limit.
Upon taking office on January 16, 2018, Governor Murphy expanded the medical program by issuing Executive Order No. 6, which ordered a 60-day review of all aspects of New Jersey’s current program, “with a focus on ways to expand access to cannabis for medical purposes.” In response to Executive Order No. 6, NJDOH released its EO 6 Report on March 23, 2018, which proposed significant changes to the existing medicinal program. In an effort to create greater patient access, the state immediately put into effect some of the recommended changes, including cutting registration and renewal fees, and expanding qualifying conditions.
On July 16, 2018, the Murphy Administration announced that the licensing application process would be opened for up to six additional vertically integrated medicinal cannabis ATCs. The NJDOH released a Notice of Request for Applications outlining the reason for issuing the licenses, eligibility rules and information required for the applications. The application period opened on August 1, 2018 and closed on August 31, 2018. Winning applicants were supposed to be selected on or before November 1, 2018 but this deadline was subsequently pushed to December due to administrative constraints. On December 17, 2018, NJDOH revealed the additional six medical cannabis ATCs it picked to add to the program. New Jersey will now have 12 vertically-integrated ATCs across the state, if these additional six applicant ATCs become operational. These six applicant ATCs now must pass background checks, provide evidence of cultivation and dispensary locations with municipal approval for each location, and comply with all regulations promulgated by the NJDOH, including safety and security requirements.
On March 25, 2019, a planned vote on legislative package including medical expansion and adult-use legalization was pulled due to a lack of votes necessary to pass the legislation through the state Senate. This setback came after significant momentum had helped to pass the bill through the appropriations and judiciary committees earlier in the month. After the package of cannabis reforms stalled, Governor Murphy announced he would be expanding the medical program through administrative action. On December 18, 2020, Governor Murphy signed A. 5981/S. 4154 into law, which facilitated the expungement of
19
low-level cannabis crimes and other offenses. On November 3, 2020, New Jersey voters passed a ballot measure amending the New Jersey Constitution to permit the use of cannabis for adults over 21 years of age. The ballot measure allowed New Jersey to regulate the growth, distribution, and sale of adult-use cannabis.
On February 22, 2021, Governor Murphy signed the New Jersey Cannabis Regulatory, Enforcement Assistance, and Marketplace Modernization Act into law, legalizing the use of cannabis by adults 21 years of age and older in New Jersey. On August 19, 2021, New Jersey’s Cannabis Regulatory Commission (“CRC”) published its first set of rules for adult-use cannabis in the state. These rules outline the details of licensing, the authority of municipalities, the operations of cannabis businesses, and the CRC’s authority over adult-use cannabis. Adult-use sales in New Jersey commenced on April 21, 2022.
In September 2023, pursuant to Resolutions 2023-143 and 2023-144, the CRC waived N.J.A.C 17:30A-10.7(e), N.J.A.C 17:30-11.5(c)(3) and N.J.A.C 17:30-11.6(b) to allow the manufacturing and dispensing of additional ingestible products as an authorized form in the medical and adult-use cannabis industries.
Pennsylvania
The Pennsylvania medical cannabis program was signed into law on April 17, 2016, under Act 16 and provided access to state residents with one or more of 17 qualifying conditions, including: epilepsy, chronic pain, and PTSD. The state originally awarded only 12 licenses to cultivate/process and 27 licenses to operate retail dispensaries (which entitled holders to up to three medical dispensary locations per retail license).
On March 22, 2018, it was announced that the final phase of the Pennsylvania medical cannabis program would initiate its rollout, which included 13 additional cultivation/processing licenses and 23 additional dispensary licenses. Additionally, the list of qualifying conditions was expanded from 17 to 21. On July 20, 2019, two more qualifying medical conditions were added, bringing the total to 23.
There are two principal license categories in Pennsylvania: (1) cultivation/processing and (2) dispensary. All cultivation/processing establishments and dispensaries must register with Pennsylvania Department of Health. Registration certificates are valid for a period of one year and are subject to annual renewals after required fees are paid and the business remains in good standing. The Pennsylvania Department of Health must renew a permit unless it determines the applicant is unlikely to maintain effective control against diversion of medical cannabis and the applicant is unlikely to comply with all laws as prescribed under the Pennsylvania medical cannabis program.
Under applicable laws, the licenses permit the license holder to cultivate, manufacture, process, package, sell and purchase medical cannabis pursuant to the terms of the licenses, which are issued by the Pennsylvania Department of Health under the provisions of Medical Marijuana Act and Pennsylvania regulations. The medical cultivation/processing licenses permit the licensee to acquire, possess, cultivate, manufacture/process into medical cannabis products and/or medical cannabis-infused products, deliver, transfer, have tested, transport, supply or sell cannabis and related supplies to medical cannabis dispensaries.
The retail dispensary licenses permit the Company to purchase cannabis and cannabis products from cultivation/processing facilities, as well as allow the sale of cannabis and cannabis products.
Maryland
The Maryland medical cannabis program was signed into law on May 2, 2013. In 2016, the Maryland Medical Cannabis Commission issued preliminary licenses to 102 dispensaries, 15 cultivators, and 15 processors; the first dispensaries opened to patients in December 2017.
Maryland has three classes of cannabis licenses: dispensaries, cultivators, and processors. Wholesaling occurs between cultivators and processors, cultivators and dispensaries, and processors and dispensaries. Originally, no one company could directly control multiple licenses of the same class, but this restriction was changed in May 2019 when Governor Hogan signed a bill that permitted a single company to own or control, including the power to manage or operate, up to four dispensaries. Dispensary locations are tied to the Senate District in which they were awarded, with the exception of applicants who were awarded dispensary and cultivation licenses. These dispensaries can be located at the discretion of the license holder. Permitted products include oil-based formulations, flower, and edibles.
In April 2018, the Maryland House and Senate approved a bill, which was later signed by Governor Hogan, that expanded the license pool, allowing for a maximum of seven additional cultivation licenses, for a total of 22, and 13 additional processing licenses, for a total of 28. In November 2022, the voters in Maryland approved adult-use cannabis as a ballot question.
20
Beginning on July 1, 2023, adults 21 or older may possess and consume up to 1.5 ounces of cannabis flower, 12 grams of concentrated cannabis, or a total amount of cannabis products that does not exceed 750 mg THC. This amount is known as the "personal use amount." Adult-use sales are expected to begin around the same time. Subsequently, in July 2023, the Maryland Cannabis Administration (the "MCA") was established to oversee both Maryland's medical and adult-use cannabis programs. As of July 1, 2023, all medical license holders that chose to convert their licenses and whose conversion applications the MCA approved became the holders of "hybrid" medical and adult-use licenses (i.e., each license issued by the MCA permits the cultivation, processing, and dispensing of both medical and adult-use cannabis).
Michigan
In November 2008, Michigan residents approved the Michigan Compassionate Care Initiative to provide a legal framework for individuals with certain debilitating medical conditions to lawfully use cannabis for medicinal purposes. In September 2016, the Michigan Legislature passed, and Governor Snyder signed into law, the Medical Marihuana Facilities Licensing Act (the “MMFLA”) and the Marihuana Tracking Act (the “MTA”) to provide a comprehensive licensing and tracking scheme, respectively, for state’s medical cannabis program.
In 2018, Michigan voters approved Proposal 1, to make cannabis legal under state and local law for adults 21 years of age or older and to control the commercial production and distribution of cannabis under a system that licenses, regulates, and taxes the businesses involved. The proposal is known as the Michigan Regulation and Taxation of Marihuana Act (the “MRTMA” and together with the Michigan Medical Marihuana Act, the MMFLA, and the MTA, the “Michigan Cannabis Laws”).
Additionally, the Michigan Department of Licensing and Regulatory Affairs (“LARA”) and the Marijuana Regulatory Agency ("MRA") have supplemented the Michigan Cannabis Laws with administrative rules and bulletins to further clarify the regulatory landscape surrounding the state’s medical and adult-use cannabis programs. The MRA is the main regulatory authority for the licensing and regulation of medical and adult-use cannabis businesses and is an agency within LARA.
Under the MMFLA, the MRA administrates five types of “state operating licenses” for medical cannabis businesses: (a) a “grower” license, including three different classes of licenses: (i) a Class A license that allows the licensee to cultivate up to 500 plants; (ii) a Class B license that allows the licensee to cultivate up to 1,000 plants; and (iii) a Class C license that allows the licensee to cultivate up to 1,500 plants, (b) a “processor” license, (c) a “secure transporter” license, (d) a “provisioning center” license and (e) a “safety compliance facility” license. Likewise, under the MRTMA, the MRA administers six types of “state licenses” for adult-use cannabis business: (a) a “marihuana grower” license, including three different classes of licenses: (i) a Class A license that allows the licensee to cultivate up to 100 plants; (ii) a Class B license that allows the licensee to cultivate up to 500 plants; and (iii) a Class C license that allows the licensee to cultivate up to 2,000 plants, (b) a “marihuana processor” license, (c) a “secure transporter” license, (d) a “marihuana retailer” license, (e) a “marihuana safety compliance facility” license, and (f) a “marihuana microbusiness” license. However, MRTMA also allows the MRA to create additional license types through the administrative rulemaking process. To date, the MRA has created four additional license types: (a) a “designated consumption establishment” license, (b) an “excess marihuana grower” license, (c) a “marihuana event organizer” license, and (d) a “temporary marihuana event” license. Importantly, each excess marihuana grower license allows an entity that holds five adult-use Class C grower licenses issued under MRTMA and at least two medical Class C grower licenses issued under the MMFLA to grow an additional 2,000 plants.
Under both MRTMA and the MMFLA, there are no stated limits on the number of licenses that can be made available on a state level; however, the MRA has discretion over the approval of applications and municipalities can pass additional restrictions, including limiting the number and type of licenses that can be issued within their jurisdiction. Additionally, a person or entity cannot possess or own both a grower/process/provisioning center and a secure transporter license or a safety compliance lab.
On February 11, 2022, Governor Whitmer signed an Executive Reorganization Order 2022-1 which modified the MRA to be called the Cannabis Regulatory Agency ("CRA"). This allowed for the CRA to have authority over Michigan's hemp processors and handlers under the Industrial Hemp Research and Development Act also shifting to the new CRA.
U.S. Hemp Regime
The Agriculture Improvement Act of 2018 (commonly known as the “2018 Farm Bill”) was signed into law on December 20, 2018. The 2018 Farm Bill, among other things, removed “hemp” (including any part of the cannabis plant containing 0.3% THC or less), its extracts, derivatives, and cannabinoids from the Controlled Substances Act definition of “marihuana”, and allows for federally sanctioned hemp production under the purview of the U.S. Department of Agriculture (the “USDA”), in coordination with state departments of agriculture that elect to have primary regulatory authority. States and Tribal governments can adopt their own regulatory plans, even if more restrictive than federal regulations, so long as the plans meet minimum
21
federal standards and are approved by the USDA. Hemp production in jurisdictions that do not choose to submit their own plans (and that do not otherwise prohibit hemp production) will be governed by USDA regulation. “Hemp” as defined in the 2018 Farm Bill, “means the plant Cannabis sativa L., and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not with a THC concentration of not more than 0.3% on a dry weight basis.”
While the 2018 Farm Bill removes hemp and hemp-derived products from the controlled substances list under the Controlled Substances Act, it does not legalize cannabidiol (“CBD”) in every circumstance. While not independently scheduled under the Controlled Substances Act, CBD, depending on the source from which it was derived and its THC concentration, can still be classified as a Schedule I substance under the Controlled Substances Act’s definition of “marihuana.” Further, although the 2018 Farm Bill creates a limited exception to this prohibition, this exception only applies if the CBD is derived from “hemp” as defined in federal law. Federal law also requires that: (i) the hemp is produced by a Licensed Producer; and (ii) in a manner consistent with the applicable federal and state regulations. CBD and other cannabinoids produced from marihuana as defined by the Controlled Substances Act remain an illegal Schedule I substance under federal law. In addition, many state laws include all CBD within definitions of cannabis and some states have policies or laws that otherwise prohibit or restrict CBD sales.
Notwithstanding the foregoing, the 2018 Farm Bill expressly preserves the FDA’s authority to regulate certain products under the FDCA and Section 351 of the Public Health Service Act. The FDA takes the position that because CBD was the subject of substantial clinical investigations that have been made public and is the active ingredient in an FDA-approved drug (Epidiolex), it is therefore illegal to add to food and CBD products are excluded from the dietary supplement definition. While there is an exception for articles that were marketed as a conventional food or dietary supplement before the new drug investigations were authorized (or the new drug was approved), the FDA has asserted that, based on available evidence, the exception does not apply to CBD. As previously mentioned, the FDA takes the position that it is unlawful under the FDCA to introduce food containing added CBD into interstate commerce, or to market CBD products as, or in, dietary supplements, regardless of whether the substances are hemp-derived. Despite FDA’s stated position, the agency has not, to date, been active in its CBD-related enforcement absent CBD products bearing aggressive therapeutic claims (e.g., claims of treatment of COVID-19, neuropathy, AIDS, diabetes, cancer, etc.). FDA could change its enforcement priorities at any time. The FDA has indicated that it will work towards providing ways for companies to seek approval from the FDA to market CBD products. In addition, options remain available for the FDA to consider whether there are circumstances in which certain cannabis-derived compounds might be permitted in a food or dietary supplement. Importantly, notwithstanding the FDA’s stated position prohibiting sales of CBD containing-foods and dietary supplements, the FDA has authority to issue a regulation allowing the use of a pharmaceutical ingredient, such as CBD, in a food or dietary supplement, even if such pharmaceutical ingredient was not previously marketed as a food or dietary ingredient prior to the initiation of clinical drug trials. Timing regarding if or when FDA might issue such a regulation is unclear.
States have also taken various approaches to the production and sale of hemp-derived food products. Many states have adopted the Uniform State Food, Drug, and Cosmetic Act, which was created in 1984 by the Association of Food and Drug Officials (the “AFDO”), the primary organization for state food and drug officials. The AFDO’s model Uniform Act includes a provision to automatically incorporate changes to the FDCA into state law. However, there is some variation between state Food, Drug, and Cosmetic Acts both because not all states have adopted this provision, and because not all states have adopted the Uniform Act. States that have adopted the Uniform Act generally prohibit the use of CBD in food and dietary supplements due to the FDA’s lack of approval for such uses of the substance, discussed above. For example, Michigan (among other states) prohibits the use of CBD in retail food and beverage products because of the FDA’s stated position. Like FDA, some states, despite having stated positions of the impermissibility of CBD foods and supplements, or any CBD products at all, enforcement is inconsistent, with some state regulators more active than others. Again, these enforcement priorities could change at any time.
Although hemp-derived CBD cannot be added or marketed in foods or dietary supplements, certain hemp derived substances, such as hemp seed oil, may be permissible in food, dietary supplements, cosmetics, and other products depending on whether the ingredients and finished products comply with the other requirements of the FDCA. For example, a substance that will be added to food is subject to premarket approval by the FDA unless it is generally recognized, among qualified experts, to be safe under the conditions of its intended use (“GRAS”). Pursuant to the FDCA, a food ingredient may be marketed in the United States under any of the following three alternative criteria: (i) if it was approved by the FDA or USDA between 1938 and 1958 for the intended use (commonly referred to as a “prior sanction”); (ii) if it is GRAS for its intended use; or (iii) pursuant to a food additive regulation promulgated by the FDA. On December 20, 2018, the FDA issued GRAS approvals for three ingredients that are derived from parts of the cannabis plant that do not contain THC or CBD – hulled hemp seeds, hemp seed protein, and hemp seed oil. These three types of products can be legally marketed in human foods without food additive approval, provided they comply with all other requirements and do not make unlawful drug claims. It is worth noting that none of these GRAS products contain CBD.
22
The 2018 Farm Bill also contemplates a significant state presence in the regulation of hemp production, as the 2018 Farm Bill empowers states, U.S. territories and Native American tribes to regulate the production and sale of hemp within their respective borders. To regulate commercial hemp production, states, U.S. territories and Native American tribes must submit plans to the USDA setting out the processes associated with how the state, territory or tribe will regulate hemp production, including how it will gather information, test, inspect and dispose of hemp and its related byproducts. The Secretary of the USDA must approve or reject these plans within sixty days of receipt. If a state, territory or tribe chooses not to submit a plan to the USDA, potential producers will be able to apply directly to the USDA for licensing approval. States, territories and tribes may also enact stricter laws than those enacted at the federal level and may ban hemp production and sale within their respective jurisdiction.
Once implemented, in jurisdictions with USDA-approved state programs, it will be a violation of state law to cultivate hemp without a registration in compliance with state law, or in the case of a state or territory without a USDA-approved program, it will be a violation of federal law to cultivate hemp without a federally issued license.
The 2018 Farm Bill was signed into law on December 20, 2018. Importantly, however, the Industrial Hemp cultivation and research provisions contained in the Section 7606 of the Agricultural Act of 2014 (the “2014 Farm Bill”) will remain in effect pending the USDA’s rulemaking process and certain provisions of the law may not yet be effective. The federal rulemaking process may take more than one year to finalize, and the 2014 Farm Bill will be repealed one year after the USDA establishes regulations governing hemp production in states lacking their own USDA-approved plans. The scope of the 2014 Farm Bill is limited to cultivation that is: (i) for research purposes (inclusive of market research, which multiple federal agencies have confirmed includes commercial sales with a research purpose); (ii) part of an “agricultural pilot program” or other agricultural or academic research; and (iii) permitted by state law. Further, the 2014 Farm Bill defines “Industrial Hemp” as the plant Cannabis sativa L., and any part of such plant, whether growing or not, with a delta-9 THC concentration of not more than 0.3% on a dry weight basis. The USDA published its final rule on January 19, 2021. The USDA’s final rule establishes a federal licensing plan for regulating U.S. hemp producers in states that do not have their own USDA-approved plans. In the absence of a state plan, U.S. hemp producers will be subject to regulation directly by the USDA unless the state prohibits U.S. hemp production. Additionally, the final rule includes requirements for maintaining information on the land where US hemp is produced, testing U.S. hemp for THC levels, disposing of plants with more than 0.3 percent THC on a dry-weight basis and licensing for U.S. hemp producers. The USDA’s final rule requires hemp producers to use a laboratory that is registered with the DEA, although the USDA is delaying enforcement of this requirement until December 31, 2022. The final rule also includes provisions for producers to dispose or remediate violative hemp plants without the use of a DEA-registered reverse distributor or law enforcement.
In November 2023, President Joseph Biden signed a stopgap funding bill that Congress passed with bipartisan support. The new law extended the 2018 Farm Bill to September 30, 2024, which had previously expired on September 30, 2023. This will allow time for Congress to draft a new Hemp Farm bill.
Regulatory Framework in Canada
Licenses and Regulatory Framework in Canada
The Cookies Canada License
The Company operates the Cookies Canada retail dispensary in Ontario, Canada through TerrAscend Canada, which holds a retail operator's license under the Cannabis License Act (Ontario). The retail operator's license permits, subject to further requirements set out in the legislation, TerrAscend Canada to possess and sell cannabis, including sales of cannabis extracts, topicals and edibles, and oils, in accordance with the applicable legislation and the regulations promulgated thereunder, subject to certain terms and conditions, subject to certain restrictions on age and time of sale.
The TerrAscend Canada Licensed Producer License
TerrAscend Canada held a standard cultivation license, standard processing license and license for sale for medical purposes under the Cannabis Act until February 9, 2023 when it opted to return its license.
Summary of Canadian Regulatory Framework
On October 17, 2018, the Cannabis Act and the Cannabis Regulations (the "Cannabis Regulations") came into force as law with the effect of legalizing the recreational adult-use of cannabis and regulating the production, distribution and sale of cannabis and cannabis derived products (both medical and adult-use) within Canada. The Cannabis Act replaced Controlled Drug and Substances Act (Canada) (the “CDSA”). Under the CDSA, the Access to Cannabis for Medical Purposes Regulations
23
(the “ACMPR”) set out a framework to provide individuals with access to cannabis for medical purposes and was the governing legislation in respect of the production, sale and distribution of medical cannabis and related oil extracts in Canada. Although ACMPR was repealed, the regulatory framework applicable to cannabis for medical purposes was substantially reproduced within the Cannabis Act with minimal changes.
The Cannabis Regulations provide a licensing and permitting scheme for activities related to cannabis setting out the following classes of licenses that authorize activities in relation to cannabis: (i) a license for cultivation; (ii) a license for processing; (iii) a license for analytical testing; (iv) a license for sale for medical purposes; (v) a license for research; and (vi) a cannabis drug license. Each license contemplates permitting the sale of cannabis within the supply chain (i.e. to other appropriate license holders or those permitted under a provincial law) but does not include the ability to sell recreational cannabis to retail consumers which is regulated by the provinces. At the end of each term of their respective licenses, a license holder must submit an application for renewal to Health Canada containing information prescribed by the Cannabis Act.
The Cannabis Regulations, among other things, outline the rules for the legal cultivation, processing, research, testing, distribution, sale, importation and exportation of cannabis and hemp in Canada.
Pursuant to the Cannabis Act, but subject to provincial or territorial restrictions, adults who are over the age of 18 are legally able to: (i) possess up to 30 grams of legal cannabis, dried or equivalent in non-dried form in public; (ii) share up to 30 grams of legal cannabis, dried or equivalent in non-dried form with other adults; (iii) buy dried or fresh cannabis and cannabis oil from a provincially licensed retailer; (iv) grow, from licensed seed or seedlings, up to four cannabis plants per residence for personal use; and (v) make cannabis products, such as food and drinks, at home as long as dangerous organic solvents are not used to create concentrated products.
In the initial stage of the regulated adult-use cannabis market, products available for sale were the same as those permitted in the medical cannabis market (dried flowers, oils and softgels and dried cannabis products). On October 17, 2019, the second phase of adult-use cannabis products, specifically, edible cannabis products, cannabis extracts, and cannabis topical products, were legalized pursuant to certain amendments to the regulations under the Cannabis Act. Edible cannabis products, cannabis extracts, and cannabis topical products, which are now available for sale, are subject to additional regulatory requirements that include supplemental marketing and advertising rules, further restrictions on labelling and packaging, rules relating to ingredients of edible cannabis products and cannabis extracts, limits on THC content, and added production facility requirements.
Further, the current regime for medical cannabis will continue to allow access to cannabis for individuals who have the authorization of their healthcare provider.
Provincial and Territorial Developments
While the Cannabis Act provides for the regulation by the Canadian federal government of, among other things, the commercial cultivation and processing of cannabis and the sale of medical cannabis, it also provides that the provinces and territories of Canada have authority to regulate certain aspects of adult-use cannabis, such as retail and distribution of adult-use cannabis, as well as the ability to alter some of the existing baseline requirements, such as increasing the minimum age for purchase and consumption, restricting places where cannabis can be consumed, and a range of other matters.
The governments of every Canadian province and territory have implemented their regulatory regimes for the distribution and sale of cannabis for adult-use purposes, and as such, regulatory regimes vary from jurisdiction to jurisdiction. In each of the provinces and territories, except for Saskatchewan, a provincial distributor is responsible for purchasing cannabis from producers and selling products to its regulated retail distribution channels. Most provinces and territories have announced a minimum age of 19 years old, except for Alberta, where the minimum age is 18, and Québec, where the minimum age is 21. In Ontario, the province in which the Company operates Cookies Canada through TerrAscend Canada, the distribution and sale of recreational cannabis is primarily governed by the Cannabis Control Act, the Cannabis License Act, and the regulations promulgated thereunder. The Ontario Cannabis Retail Corporation is the wholesale distributor of cannabis and conducts all online sales in Ontario.
With respect to retail sales of cannabis, other than online sales, the provincial and territorial regulations in Prince Edward Island, Nova Scotia, New Brunswick and Quebec allow only for government-run cannabis stores, while the provincial and territorial regulations in Ontario, Alberta, Newfoundland and Labrador, Nunavut, Yukon, Saskatchewan and Manitoba leave the retail sale of cannabis, other than online sales, to the private sector. In British Columbia, Northwest Territories and New Brunswick, provincial and territorial regulations allow for a hybrid model in which both public and private stores can operate. In addition, in Ontario, British Columbia, Newfoundland, Northwest Territories, New Brunswick, Nova Scotia, Prince Edward Island, Quebec and Yukon, the provincial body is solely responsible for online sales.
24
Most recently, following extensive engagement with stakeholders, universities, researchers, health authorities, cannabis industry associations, cannabis license holders, provinces, territories and the public, the Canadian federal government announced the following amendments to the Cannabis Act and its regulations:
Broadening the educational qualifications for the Head of Laboratory, a position that is required for an analytical testing license and is responsible for all cannabis testing activities that occur at the licensed site.
Compliance
TerrAscend believes it is in compliance with all applicable laws in the jurisdictions it operates including all state laws, the cannabis licensing framework of Maryland, Pennsylvania, New Jersey, California, and Michigan, and all provincial laws and the regulations in Ontario, and at the time of license return at the request of the Company, TerrAscend Canada believed it was in compliance with all applicable federal, provincial and territorial laws and regulations, including the Cannabis Act and the Cannabis Regulations. There are no known current incidences of material non-compliance, citations or notices of violations outstanding which may have an impact on the Company’s licenses, business activities or operations where it operates. Notwithstanding the foregoing, like all businesses, the Company may from time to time experience incidences of non-compliance with applicable rules and regulations in the jurisdictions in which the Company operates, and such non-compliance may have an impact on the Company’s licenses, business activities or operations. However, the Company takes steps to minimize, disclose and remedy all incidences of non-compliance which may have an impact on the Company’s licenses, business activities or operations where the Company operates. Notwithstanding the fact that various states in the United States have implemented medical cannabis laws or have otherwise legalized the use of cannabis, the use of cannabis remains illegal under U.S. federal law for any purpose, by way of the Controlled Substances Act.
Available Information
The Company’s website address is www.terrascend.com. Through this website, the Company’s filings with the SEC, including its annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and all amendments to such reports, will be accessible (free of charge) as soon as reasonably practicable after materials are electronically filed with or furnished to the SEC, and copies thereof are electronically filed on the System for Electronic Document Analysis and Retrieval + ("SEDAR+") in Canada. Information contained on or accessible through the Company's website is not a part of this Annual Report, and the inclusion of the Company's website address in this Annual Report is an inactive textual reference only.
The SEC maintains an internet site (http://www.sec.gov) that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC.
ITEM 1A. RISK FACTORS
The following risks should be carefully considered when deciding whether to make an investment in the Company. Some of the following factors are interrelated and, consequently, investors and readers should treat such risk factors as a whole. These risks and uncertainties are not the only ones that could affect the Company, and additional risks and uncertainties not currently known to the Company, or that it currently considers not to be material, may also impair the business, financial condition and results of operations of the Company and/or the value of its securities. If any of the following risks or other risks occur, they could have a material adverse effect on the Company's business, financial condition and results of operations and/or the value of the Company’s securities. There is no assurance that any risk management steps taken by the Company will avoid future loss due to the occurrence of the risks described below, or other unforeseen risks.
25
Regulatory and Legal Risks Related to the Company’s Business and the Cannabis Industry
There is a substantial risk of regulatory or political change with respect to cannabis, which could have a material adverse effect on the Company and its business.
In the United States, the operations of TerrAscend and its subsidiaries are subject to a variety of laws, including, among other things, state and local regulations and guidelines relating to the cultivation, manufacture, management, transportation, distribution, sale, storage and disposal of cannabis. Changes to such laws, regulations and guidelines due to matters beyond the control of the Company may cause adverse effects to the Company’s business, financial condition and result of operations. Local, state and federal laws and regulations governing cannabis for medicinal and adult-use purposes are broad in control and are subject to evolving interpretations, which could require the Company to incur substantial costs associated with bringing the Companyʼs operations into compliance. In addition, violations of these laws, or allegations of such violations, could disrupt the Companyʼs operations and result in a material adverse effect on its financial performance. It is beyond the Companyʼs scope to predict the nature of any future change to the existing laws, regulations, policies, interpretations or applications, nor can the Company determine what effect such changes, when and if promulgated, could have on the Companyʼs business.
In the United States, the production and sale of cannabis is prohibited according to the Controlled Substances Act. If cannabis is re-classified or there are changes in U.S. controlled substance laws and regulations, such changes could have a material adverse effect on our business, financial condition, and results of operations. If cannabis is re-classified as a Schedule II or lower controlled substance, the resulting re-classification would result in the need for approval by FDA and DEA. As a result of such a re-classification, the manufacture, importation, exportation, domestic distribution, storage, sale and use of such products could become subject to a significant degree of regulation by the FDA and DEA. In that case, we may be required to be registered to perform these activities and have the security, control, recordkeeping, reporting and inventory mechanisms required by the DEA to prevent drug loss and diversion. Obtaining the necessary registrations may result in delay of the manufacturing or distribution of our products. The DEA conducts periodic inspections of registered establishments that handle controlled substances. Failure to maintain compliance could have a material adverse effect on our business, financial condition and results of operations. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to restrict, suspend or revoke those registrations. In certain circumstances, violations could lead to criminal proceedings.
Furthermore, should the United States federal government legalize cannabis, it is possible that the FDA would seek to regulate it under the Food, Drug and Cosmetics Act of 1938. Additionally, the FDA may issue rules and regulations, including good manufacturing practices related to the growth, cultivation, harvesting and processing of medical and adult-use cannabis. Clinical trials may be needed to verify efficacy and safety of medical cannabis products. It is also possible that the FDA would require that facilities where cannabis is grown or manufactured register with the agency and comply with certain federally prescribed regulations. In the event that some or all of these regulations are imposed, the impact on the cannabis industry is uncertain and could include the imposition of new costs, requirements, and prohibitions. If we are unable to comply with the regulations or registration as prescribed by the FDA, it may have an adverse effect on our business, operating results, and financial condition.
In Canada, the Cannabis Act was established on October 17, 2018 along with various related regulations. The cultivation, processing, distribution and sale of cannabis, among other things, remains subject to extensive regulatory oversight in Canada under the Cannabis Act. It is possible that these statutory requirements, including any new regulations that are subsequently issued, could significantly and adversely affect the business, financial condition and results of operations of the Company.
Furthermore, the distribution of adult-use cannabis is the responsibility of the respective provincial and territorial governments. There is no guarantee that provincial and territorial legislation regulating the distribution and sale of cannabis for adult-use purposes will continue according to their current terms, that they will not be materially amended or that such regimes will create the growth opportunities currently anticipated by the Company.
In addition, government policy changes or public opinion may also influence the regulation of the cannabis industry in Canada, the United States or elsewhere. A negative shift in the public’s perception of medical or adult-use cannabis in Canada, the United States or any other applicable jurisdiction could affect future legislation or regulation. Among other things, a shift could cause state and local jurisdictions to abandon initiatives or proposals to legalize medical or adult-use cannabis, thereby limiting the number of new state or provincial jurisdictions into which the Company could expand. Any inability to fully implement the Companyʼs expansion strategy may have a material adverse effect on the Companyʼs business, financial condition and results of operations.
26
Compliance with regulations regarding cannabis is difficult, because the regulation of cannabis is uncertain and frequently changes. The Company's failure to comply with applicable laws regarding cannabis may adversely affect the Company's business.
Achievement of the Companyʼs business objectives is contingent, in part, upon compliance with regulatory requirements enacted by governmental authorities and obtaining all regulatory approvals, where necessary, for the production and sale of its products. The Company cannot predict the impact of the compliance regime that the applicable regulatory bodies in the United States and Canada are implementing that may affect the business of the Company. Similarly, the Company cannot predict the time required to secure all appropriate regulatory approvals for its products, or the extent of testing and documentation that may be required by governmental authorities. The impact of governmental compliance regimes, any delays in obtaining, or failure to obtain regulatory approvals may significantly delay or impact the development of markets, products and sales initiatives and could have a material adverse effect on the business, results of operations and financial condition of the Company.
The Company will incur ongoing costs and obligations related to regulatory compliance. Failure to comply with regulations may result in additional costs for corrective measures, penalties or restrictions on the Companyʼs operations. In addition, changes in regulations, more vigorous enforcement thereof or other unanticipated events could require extensive changes to the Companyʼs operations, result in increased compliance costs or give rise to material liabilities, which could have a material adverse effect on the business, results of operations and financial condition of the Company.
The cannabis industry is subject to extensive controls and regulations, which may significantly affect the financial condition of the Company. The marketability of any product may be affected by numerous factors that are beyond the control of the Company and which cannot be predicted, such as changes to government regulations, including those relating to taxes and other government levies which may be imposed. Changes in government levies, including taxes, could reduce the Companyʼs earnings and could make future capital investments or the Companyʼs operations uneconomic. The industry is also subject to numerous legal challenges, which may significantly affect the financial condition of market participants and which cannot be reliably predicted.
The Company’s business relies heavily on its ability to obtain and maintain required licenses, and failure to do so may adversely affect its business.
The Companyʼs ability to cultivate, manufacture and sell medicinal and adult-use cannabis in certain U.S. states and Canada is dependent on its ability to maintain licenses with applicable regulators for the cultivation, manufacture, and sale of cannabis. Failure to comply with the requirements of its licenses or any failure to maintain its licenses could have a material adverse impact on the business, financial condition and operating results of the Company.
The Company and its subsidiaries, as applicable, will apply for, as the need arises, all necessary licenses and permits for the activities it expects to conduct in the future. However, the ability of the Company or its subsidiaries to obtain, maintain or renew any such licenses and permits on acceptable terms is subject to changes in regulations and policies and at the discretion of the applicable authorities or other governmental agencies in each jurisdictions.
In certain jurisdictions, the cannabis laws and regulations limit, not only the number of cannabis licenses issued, but also the number of cannabis licenses that one may own. The Company believes that, where such restrictions apply, it may still capture significant share of revenue in the market through wholesale sales, exclusive marketing relations, provision of management or support services, franchising and similar arrangement with other operators. Nevertheless, limitations on the acquisition of ownership of additional licenses within certain states or enforcement by regulators in certain states against such services arrangements may limit the Companyʼs ability to grow organically or increase its market share in such states.
As a cannabis business, the Company is subject to unfavorable tax treatment under U.S. federal income tax law.
Tax risk is the risk of changes in the tax environment that would have a material adverse effect on the Companyʼs business, results of operations, and financial condition. Currently, U.S. state licensed cannabis businesses are assessed a comparatively high effective U.S. federal income tax rate due to Section 280E of the Internal Revenue Code of 1986, as amended (the "Code"), which prohibits businesses associated with trafficking in controlled substances (within the meaning of Schedule I and II of the Controlled Substances Act) from deducting certain expenses. The IRS has invoked Section 280E of the Code in tax audits against various cannabis businesses in the United States that are permitted under applicable U.S. state laws. Although the IRS issued a clarification allowing the deduction of certain expenses, the scope of such items is interpreted very narrowly, and the bulk of operating costs and general administrative costs are not permitted to be deducted. While there are currently several pending cases before various U.S. administrative and federal courts challenging these restrictions, there is no guarantee that these courts will issue an interpretation of Section 280E of the Code favorable to cannabis businesses. Given these facts, the
27
impact of any such challenges cannot be reliably estimated; however, it may be significant to the financial condition and/or the overall operations of the Company.
The Company is preparing to amend its U.S. federal income tax returns for 2020, 2021, and 2022 based on legal interpretations that challenge its tax liability under 280E of the Code. The Company has taken the position that it does not owe taxes attributable to the application of Section 280E of the Code. If the Company’s tax positions were to be challenged by U.S. federal, state, or local or non-U.S. tax jurisdictions, the Company may not be wholly successful in defending its tax filing positions. The Company records reserves for unrecognized tax benefits based on its assessment of the probability of successfully sustaining tax filing positions. Management exercises significant judgment when assessing the probability of successfully sustaining the Company’s tax filing positions, and in determining whether a contingent tax liability should be recorded and, if so, estimating the amount. If the Company’s tax filing positions are successfully challenged, payments could be required that are equal to the reserved amounts which could be significant to the Company’s financial condition or results of operations.
On October 6, 2022, President Joseph Biden requested that the Secretary of HHS and the Attorney General to initiate a review as to how cannabis is currently scheduled under federal law. In August 2023, following a review by the FDA, the Secretary of HHS issued a recommendation to the DEA that cannabis be moved to Schedule III under the Controlled Substances Act. In December 2023, the DEA confirmed it is currently conducting its review. If cannabis is moved to Schedule III from Schedule I under the Controlled Substances Act, it could end the effect of Section 280E of the Code on some or all of the Company’s operations. However, any such change from Schedule I to Schedule III is beyond the control of the Company and cannot be predicted.
The Company may be at a higher risk of an IRS audit
There may be a greater likelihood that the IRS will audit the tax returns of cannabis-related businesses and any such IRS audit may require significant management attention. Additionally, the Company plans to file refund claims for several of its subsidiaries, which may also increase the likelihood of an audit. Any such audit of the Company’s tax returns could result in it being required to pay additional tax, interest and penalties, as well as incremental accounting and legal expenses, which could be material.
If the Company is or becomes a “passive foreign investment company,” its U.S. investors may suffer adverse tax consequences.
Generally, for any taxable year, if at least 75% of the Company’s gross income is passive income, or at least 50% of the value of the Company’s assets (generally determined based on a weighted quarterly average) is attributable to assets that produce, or are held for the production, of passive income, the Company will be a “passive foreign investment company” (“PFIC”) for U.S. federal income tax purposes. For purposes of these tests, passive income generally includes dividends, interest, certain gains from the sale of investment property, and certain rents and royalties, and passive assets generally include cash. Additionally, the Company generally will be treated as directly holding and receiving its proportionate share of the assets and income, respectively, of any corporation in which it owns, directly or indirectly, 25% of its stock by value. If the Company is a PFIC for any taxable year, certain U.S. investors may suffer adverse tax consequences, including ineligibility for preferential tax rates on capital gains or dividends, interest charges on certain taxes treated as deferred, and additional tax reporting requirements.
The Company’s PFIC status generally will depend on the nature and composition of the Company’s income and assets and the value of the Company’s assets (which generally will be determined based on the fair market value of each asset, with the value of goodwill determined in large part by reference to the market value of the Company’s stock from time to time, which may be volatile). If the Company’s market capitalization declines while it holds a substantial amount of cash for any taxable year, the Company may be a PFIC for such taxable year. The manner and timeframe in which the Company spends the cash it raises in any offering, the transactions it enters into, and how the Company’s corporate structure may change in the future will affect the nature and composition of the Company’s income and assets. Based on the nature and composition of the Company’s income and assets and the value of the Company’s assets, including goodwill, the Company believes that it was not a PFIC for its taxable year ended December 31, 2023. Because PFIC determination is a factual determination made annually after the end of each taxable year by applying principles and methodologies that in some circumstances are unclear and subject to varying interpretation, there can be no assurance that the Company will not be a PFIC for any taxable year, and the Company’s U.S. counsel expresses no opinion with respect to the Company’s PFIC status for any taxable year. U.S. investors should consult their own tax advisors regarding the PFIC rules’ impact in their particular circumstances.
If the Company is a PFIC for any taxable year, the tax consequences that would apply if U.S. investors were able to make a valid “qualified electing fund” (“QEF”) election would be different. At this time, the Company does not expect to provide U.S.
28
investors with the information necessary for them to make a QEF election if the Company is a PFIC for any taxable year. U.S. investors should assume that a QEF election will not be available with respect to the Company’s stock.
If the Company (or any of its non-U.S. subsidiaries) is a “controlled foreign corporation,” certain of its U.S. investors may suffer adverse tax consequences.
If a “United States person” for U.S. federal income tax purposes is treated as owning (directly, indirectly, or constructively) at least 10% of the total value or total combined voting power of the Company’s stock, such person may be treated as a “United States shareholder” (each, a "U.S. Shareholder") with respect to each “controlled foreign corporation” (“CFC”) in the Company’s group (if any). A non-U.S. corporation will be a CFC if U.S. Shareholders own (directly, indirectly, or constructively) more than 50% of the total value or total combined voting power of the stock of the non-U.S. corporation. Because the Company’s group includes one or more U.S. corporate subsidiaries, certain of its current or future non-U.S. corporate subsidiaries may be treated as CFCs (regardless of whether the Company is treated as a CFC). A U.S. Shareholder of a CFC may be required to annually report and include in its U.S. taxable income its pro rata share of the CFC’s “Subpart F income,” “global intangible low-taxed income,” and investments of earnings in U.S. property (regardless of whether the CFC makes any distributions to its shareholders). Additionally, an individual U.S. Shareholder with respect to a CFC generally will not be allowed certain tax deductions or foreign tax credits that would be allowed to a corporate U.S. Shareholder. A failure to comply with CFC reporting obligations may subject a U.S. Shareholder to significant monetary penalties and prevent the statute of limitations from running with respect to the U.S. Shareholder’s U.S. federal income tax return for the taxable year in which reporting was due. There can be no assurance that the Company will assist its U.S. investors in determining whether it (or any of its current or future non-U.S. subsidiaries) is treated as a CFC or whether such U.S. investors are treated as U.S. Shareholders with respect to any such CFC, or that the Company will furnish to any such U.S. Shareholders information that may be necessary to comply with their CFC reporting and tax paying obligations. U.S. investors should consult their own tax advisors regarding the CFC rules’ impact in their particular circumstances.
The Company’s ability to use its U.S. net operating loss carryforwards to offset its future U.S. taxable income may be subject to limitations.
The Company’s U.S. federal net operating loss carryforwards (“NOLs”) generated in taxable years beginning before January 1, 2018 may be carried forward for 20 years. The Company’s U.S. federal NOLs generated in taxable years beginning after December 31, 2017 may be carried forward indefinitely, but the utilization of such NOLs is limited. In addition, under Section 382 of the Code, a corporation that undergoes an “ownership change” (generally defined as a greater than 50% change (by value) in its stock ownership over a three-year period) is subject to limitations on its ability to utilize its pre-change U.S. federal NOLs to offset its future U.S. taxable income. If the Company has undergone an ownership change in the past, or if future changes in its stock ownership result in an ownership change, which may be outside of the Company's control, its ability to utilize its U.S. federal NOLs may be limited by Section 382 of the Code. It is uncertain if and to what extent U.S. states will conform to U.S. federal income tax law with respect to the treatment of NOLs. As a result, the Company’s ability to use its U.S. NOLs to offset its future U.S. taxable income may be subject to limitations, which could increase its tax liability and decrease its cash flow.
Tax and accounting requirements may change or be interpreted in ways that are unforeseen to the Company, and the Company may face difficulty or be unable to implement and/or comply with any such changes or interpretations.
The Company is subject to numerous tax and accounting requirements, and changes in existing rules or practices, varying interpretations of current rules or practices, or enactments of new rules or practices could have a significant adverse effect on the Company’s financial results, the manner in which the Company conducts its business, or the marketability of any of its products. For instance, the recently enacted Inflation Reduction Act imposes, among other rules, a 15% minimum tax on the book income of certain large corporations and a 1% excise tax on certain corporate stock repurchases. In many countries, including the United States, the Company is subject to transfer pricing and other tax regulations designed to ensure that appropriate levels of income are reported as earned and are taxed accordingly. Although the Company believes that it is in substantial compliance with all applicable regulations and restrictions, it is subject to the risk that governmental authorities could audit its transfer pricing and related practices and assert that additional taxes are owed or that various jurisdictions could assert that the Company should file tax returns in jurisdictions where it does not file and subject it to additional tax. In the future, the geographic scope of the Company’s business may expand, and such expansion will require the Company to comply with the tax laws and regulations of additional jurisdictions. Requirements as to taxation varies substantially among jurisdictions. Complying with the tax laws and regulations of these jurisdictions can be time-consuming and expensive and could potentially subject the Company to penalties and fees in the future if it failed to comply. In the event that the Company failed to comply with applicable tax laws and regulations, this could have a material adverse effect on its business, financial condition, and results of operations.
29
Cannabis remains illegal under U.S. federal law, and enforcement of cannabis laws could change. The Company may be subject to action by the U.S. federal government due to its involvement with cannabis in the United States, and such action could materially adversely affect the Company’s business.
While some states in the United States. have legalized the use and sale of cannabis in some form, it remains illegal under U.S. federal law. On January 4, 2018, then-U.S. Attorney General Jeff Sessions issued a memorandum to U.S. Attorneys which rescinded previous guidance from the DOJ specific to cannabis enforcement in the United States, including the Cole Memorandum, which stated that the DOJ would not prioritize the prosecution of cannabis-related violations of U.S. federal law in jurisdictions that had enacted laws legalizing medical cannabis in some form and had implemented strong and effective regulatory and enforcement systems. With the Cole Memorandum rescinded, U.S. federal prosecutors have greater discretion in determining whether to prosecute medical cannabis-related violations of U.S. federal law, though there was never such a policy statement in relation to United States and its territories with adult-use cannabis programs. Because the Company engages in cannabis-related activities in the United States, an increase in federal enforcement efforts with respect to current U.S. federal laws applicable to cannabis could cause financial damage to the Company. In addition, the Company is at risk of being prosecuted under U.S. federal law and having its assets seized.
The Companyʼs exposure to U.S. cannabis related activities are as follows:
|
|
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Current assets |
|
|
|
|
101,369 |
|
|
|
98,704 |
|
Non-current assets |
|
|
|
|
562,916 |
|
|
|
577,659 |
|
Current liabilities |
|
|
|
|
204,455 |
|
|
|
124,777 |
|
Non-current liabilities |
|
|
|
|
206,123 |
|
|
|
215,605 |
|
|
For the years ended |
|
||||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Revenue, net |
|
|
316,257 |
|
|
|
246,571 |
|
|
|
190,847 |
|
Gross profit |
|
|
159,349 |
|
|
|
100,941 |
|
|
|
110,783 |
|
Income (loss) from operations |
|
|
49,145 |
|
|
|
(4,994 |
) |
|
|
26,503 |
|
Net loss attributable to controlling interest |
|
|
(73,541 |
) |
|
|
(322,461 |
) |
|
|
(2,506 |
) |
Violations of any U.S. federal laws and regulations could result in significant fines, penalties, administrative sanctions, convictions or settlements arising from civil proceedings conducted by either the U.S. federal government or private citizens, or criminal charges, including, but not limited to, disgorgement of profits, cessation of business activities, civil forfeiture or divestiture. Any property owned by participants in the cannabis industry that is either used in the course of conducting or comprises the proceeds of a cannabis business could be subject to seizure by law enforcement and subsequent civil asset forfeiture. Even if the owner of the property were never charged with a crime, the property in question could still be seized and subject to an administrative proceeding by which, with minimal process, it could become subject to forfeiture. This could have a material adverse effect on the Company, including its reputation and ability to conduct business, the listing of its securities on various stock exchanges, its financial position, operating results, profitability or liquidity or the market price of its publicly traded shares. In addition, it is difficult for the Company to estimate the time or resources that would be needed for the investigation of any such matters or its final resolution because, in part, the time and resources that may be needed are dependent on the nature and extent of any information requested by the applicable authorities involved, and such time or resources could be substantial.
Unlike in Canada which has federal legislation uniformly governing the cultivation, distribution, sale and possession of cannabis under the Cannabis Act, investors are cautioned that in the United States, cannabis is largely regulated at the state level. Notwithstanding the permissive regulatory environment of cannabis at the state level, cannabis continues to be categorized as a controlled substance under the Controlled Substances Act and as such, is in violation of federal law in the United States. Further, there can be no assurance that state laws legalizing and regulating the sale and use of cannabis will not be repealed or overturned, or that local governmental authorities will not limit the applicability of state laws within their respective jurisdictions. It is also important to note that local and city ordinances may strictly limit and/or restrict the retailing of cannabis in a manner that will make it extremely difficult or impossible to transact business in the cannabis industry.
As stated above, the U.S. Congress has passed appropriations bills in each fiscal year since FY2015, that prevents the federal government from using congressionally appropriated funds to enforce federal cannabis laws against regulated medical cannabis actors operating in compliance with state and local law. The continuing resolution contains, among other things, the RBA,
30
which prevents the federal government from using congressionally appropriated funds to enforce federal cannabis laws against regulated medical cannabis actors operating in compliance with state medical cannabis laws.
Additionally, it is important to note that the appropriations protections only apply to medical cannabis operations and provide no protection against businesses operating in compliance with a state’s adult-use cannabis laws.
The DOJ or a federal prosecutor could allege that the Company, or its board of directors (the "Board of Directors" or the "Board") and, potentially its shareholders, “aided and abetted” violations of federal law by providing finances and services to its operating subsidiaries. Under these circumstances, it is possible that a federal prosecutor would seek to seize the assets of the Company and to recover the “illicit profits” previously distributed to shareholders resulting from any of the foregoing financing or services. In these circumstances, the Company’s operations could cease, shareholders of the Company may lose their entire investment and directors, officers and/or shareholders of the Company may be left to defend any criminal charges against them at their own expense and, if convicted, be sent to federal prison. Violations of any federal laws could result in significant fines, penalties, administrative sanctions, convictions or settlements arising from civil proceedings conducted by either the federal government or private citizens, or criminal charges, including, but not limited to, disgorgement of profits, cessation of business activities or divestiture. This could have a material adverse effect on the Company, including its reputation and ability to conduct business, its holding (directly or indirectly) of cannabis licenses in the United States, the listing of its securities on any securities exchanges, its financial position, operating results, profitability or liquidity or the market price of its listed securities. An investor’s contribution to and involvement in the Company’s activities may result in federal civil and/or criminal prosecution, including forfeiture of an entire investment.
Although the 2018 Farm Bill, among other things, removes hemp from the controlled substances list under the Controlled Substances Act, it does not legalize CBD generally. In particular, the 2018 Farm Bill preserves the FDAʼs authority to regulate products containing cannabis or cannabis-derived compounds. Pursuant to a statement released December 20, 2018, Frequently Asked Questions on the FDAʼs website, and numerous public statements, the FDA has taken the position that all CBD is a drug ingredient and therefore illegal to add to food or health products without the FDA's approval or further action. The FDA considers products containing CBD or other cannabis-derived compounds the same as any other FDA-regulated products and takes the position that they are subject to the same authorities and requirements as similarly regulated products, including but not limited to required approvals for food ingredients and dietary supplements based on safety standards. Importantly, the FDA has taken the position that it is unlawful under the FDCA to introduce food containing added CBD into interstate commerce, or to market CBD products as, or in, food or dietary supplements, regardless of whether the substances are hemp derived. The FDA has however indicated that it will work towards providing ways for companies to seek approval from the FDA to market CBD products. Further, many state criminal laws and food and drug laws prohibit or restrict the production and/or sale of hemp-derived CBD products. The Companyʼs U.S. hemp operations were subject to FDA oversight. There is no guarantee that the Company would be able to obtain necessary approval from regulatory authorities for its products in the United States.
TerrAscendʼs activities and operations in the United States are, and will continue to be, subject to evolving regulation by governmental authorities. The approach to the enforcement of cannabis laws may be subject to change or may not proceed as previously outlined. The USDA will promulgate additional rules governing the production of hemp in the United States, with many states in the process of amending state laws to regulate hemp production and the sale of hemp-derived products within their borders. In addition, the FDA is expected to make determinations as to how CBD products will be regulated and is expected to issue a substantial change in its regulation of dietary supplements generally. Accordingly, there are significant changes in both federal and state law that may materially impact the Companyʼs operations.
The Company’s business is subject to applicable anti-money laundering laws and regulations and have restricted access to capital markets, banking and other financial services, which may adversely affect its business.
Since the use of cannabis is currently illegal under U.S. federal law, and in light of considerations related to money laundering and other cannabis related criminality in the U.S. banking industry, U.S. banks have been reluctant to accept or deposit funds from businesses involved with the cannabis industry. Consequently, businesses involved in the cannabis industry often have difficulty finding banks willing to accept its business. Likewise, cannabis businesses have limited access, if any, to credit card processing services. As a result, cannabis businesses in the United States are largely cash-based. This complicates the implementation of financial controls and increases security issues. Furthermore, the Company maintains domestic cash deposits in Federal Deposit Insurance Corporation (“FDIC”) insured banks that exceed the FDIC insurance limits. Bank failures, events involving limited liquidity, defaults, non-performance, or other adverse developments that affect financial institutions, or concerns or rumors about such events, may lead to liquidity constraints. There can be no assurance that our deposits in excess of the FDIC or other comparable insurance limits will be backstopped by the United States government, or that any bank or financial institution with which we do business will be able to obtain needed liquidity from other banks, government institutions, or by acquisition in the event of a failure or liquidity crisis.
31
While the Company is not able to obtain financing in the United States from traditional banks or other certain federally regulated entities, the Company has been able to access equity financing through private markets in both the United States and Canada. Commercial banks, private equity firms, and venture capital firms have approached the cannabis industry cautiously to date. However, there are increasing numbers of high-net-worth individuals and family offices that have made meaningful investments in companies and businesses similar to the Company. There is neither a broad nor deep pool of institutional capital that is available to cannabis license holders and license applicants. There can be no assurance that additional financing, if raised privately, will be available to the Company when needed or on terms which are acceptable to the Company. The Companyʼs inability to raise financing to fund its operations, capital expenditures or acquisitions could limit its growth and may have a material adverse effect upon future profitability.
Under the U.S. federal law, financial transactions in the United States involving proceeds generated by cannabis-related conduct can form the basis for prosecution. The FinCEN division of the U.S. Department of Treasury has provided guidance for how financial institutions can provide services to the cannabis-related businesses consistent with the obligations under the Bank Secrecy Act.
Previously, the DOJ directed its federal prosecutors to consider the federal enforcement priorities enumerated in the Cole Memorandum when determining whether to charge institutions or individuals with any of the financial crimes described above based upon cannabis-related activity. In January 2018, the DOJ revoked the Cole Memorandum and related memorandum. While the impact remains unclear, the revocation has created uncertainty. For instance, federal prosecutors may increase enforcement activities against institutions or individuals who are engaged in financial transactions related to cannabis activities, or there may be a negative impact to the continuation of financial services in the United States with regard to cannabis-related activities. Consequently, businesses involved in the regulated cannabis industry may experience difficulties establishing banking relationships, and such difficulties may increase over time. If the Company were to experience any inability to access financial services in the United States, or have its existing financial services impacted, including its current bank accounts, this would have a direct impact on the ability for the Company to operate its businesses. This impact would increase the Companyʼs operating costs, and pose additional operational, logistical, and security challenges that could impede its inability to implement its business plans.
The U.S. federal prohibitions on the sale of cannabis may result in the Company and its partners being restricted from accessing the U.S. banking system and they may be unable to deposit funds in federally insured and licensed banking institutions. Banking restrictions could be imposed due to the Companyʼs banking institutions not accepting payments and deposits. The Company is at risk that any bank accounts it has could be closed at any time and result in increased costs for the Company. The Companyʼs activities in the United States, and any proceeds thereof, may be considered proceeds of crime due to the fact that cannabis remains federally illegal in the United States This may restrict the ability of the Company to declare or pay dividends, effect other distributions or subsequently repatriate such funds back to Canada. Furthermore, while the Company has no current intention to declare or pay dividends on its Common Shares in the foreseeable future, the Company may decide or be required to suspend declaring or paying dividends without advance notice and for an indefinite period of time. The guidance provided in the FinCEN Memorandum as described above may change depended on the position of the U.S. government administration at any given time and is subject to revision or retraction in the future, which may restrict the Companyʼs access to banking services.
The Company operates in a highly regulated sector and may not always succeed in complying fully with applicable regulatory requirements in the jurisdictions which it operates, which could negatively affect its business.
Given the complexity of the U.S. regulation of the cannabis industry, certain requirements may prove to be excessively onerous or otherwise impractical for the Company to comply with. This may result in the exclusion of certain business opportunities from the list of possible transactions that the Company would otherwise consider. Further, U.S. laws and regulations at the local, state, and federal levels which apply to the cannabis industry are continually changing, and it is difficult to determine if future changes could detrimentally affect the operations of the Company. Given the broad scope of cannabis laws and regulations, these are subject to evolving interpretations. This continued evolution could require the Company to incur substantial costs associated with compliance or alter its business plan. In addition, violations of these laws, or allegations of such violations, could disrupt the Companyʼs businesses and result in a material adverse effect on its operations.
The Companyʼs continued compliance with regulatory requirements enacted by government authorities and obtaining all regulatory approvals, where necessary, for the sale of its products, including maintain and renewing all applicable licenses, is crucial to the successful execution of the Companyʼs strategies. The cannabis industry is an emerging industry in the United States, and the Company cannot forecast the impact of each compliance regime to which they will be subject. Similarly, the Company cannot predict its ability to secure all appropriate regulatory approvals for any of its products, or the extent of testing or related documentation that may be required by governmental authorities. Delays in obtaining, or failure to obtain, regulatory approvals may significantly delay or impact the development of markets, products and sales initiatives and could have adverse
32
effect on the business, financial condition, and operating results of the Company. Without limiting the foregoing, the Companyʼs failure to comply with the requirements of any underlying licenses or any failure to maintain any underlying licenses would have a material adverse impact on its business, financial condition, and operating results. It is uncertain whether any required licenses for the operation of the Companyʼs business will be extended or renewed in a timely manner, if at all, or that if they are extended or renewed, that the licenses will be extended or renewed on the same or similar terms.
Furthermore, Internet websites are visible by people everywhere, not just in jurisdiction where the activities described therein are considered legal. As a result, to the extent that the Company sells services or products via web-based links targeting only jurisdictions in which such sales or services are compliant with state law, the Company may face legal action in other jurisdictions which are not the intended object of any of the Company's marketing efforts for engaging in any web-based activity that results in sales into such jurisdictions deemed illegal under applicable laws.
Regulatory restrictions on ownership outside of the control of the Company may have a material adverse impact on the Company's operations in certain jurisdictions.
The Company's business is subject to oversight by regulators in each market that the Company operates in. Regulators may limit the type or number of licenses that a single company may own. As a result, the Company may be restricted from participating in certain cannabis businesses in specific markets as a result of ownership or control of other cannabis licenses. As an example, certain markets may limit the size of a company's cultivation space, limit the number of dispensaries a single-company may own, or may obligate a company to offer certain products, like medical cannabis, to consumers.
The Company faces further risk associated with restrictions on licenses as a result of the Company's inability to control public company shareholder ownership, and therefore the Company may inadvertently have certain restrictions placed on its ability to operate as a result of third-party ownership. Restrictions placed on the Company's ability to own specific licenses or assets in specific markets, or complexities arising from third-party ownership thresholds may materially adversely impact the Company's ability to compete in a specific market or may cause other regulatory delays in receiving regulatory approval for certain activities.
Failure to comply with privacy or medical practice laws and regulations may result in impacts to operations, monetary fines or litigation.
The Company maintains an array of sensitive information, including confidential business and personal information in connection with its operations, and is subject to laws and regulations governing the privacy and security of such information. The global data protection landscape is rapidly evolving, and the Company may be affected by or subject to new, amended, or existing laws and regulations in the future, including as its operations continue to expand and the Company operates in additional markets. Each market may be subject to differing laws or legal interpretations, which adds to the complexity of collecting, using, disclosing, and processing personal data, and emerging cannabis markets may make changes to their legal frameworks.
In the United States, there are numerous federal and state privacy and data security laws and regulations governing the collection, use, disclosure, and protection of personal information, including federal and state health information privacy laws, federal and state security breach notification laws and federal and state consumer protection laws. Each of these laws is subject to varying interpretations and constantly evolving. For example, certain state laws govern the privacy and security of personal information, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Additionally, in the United States, the Health Insurance Portability and Accountability Act (“HIPAA”) imposes privacy and security requirements and breach reporting obligations with respect to individually identifiable health information upon “covered entities” (health plans, health care clearinghouses and certain health care providers), and their respective business associates, individuals or entities that create, received, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity. HIPAA mandates the reporting of certain breaches of health information to the HHS, affected individuals and if the breach is large enough, the media. Entities that are found to be in violation of HIPAA as the result of a breach of unsecured protected health information, a complaint about privacy practices or an audit by HHS, may be subject to significant civil, criminal, and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. In addition, provisions of the Americans with Disabilities Act require confidential treatment of employee medical records.
In Canada, the Personal Information Protection and Electronics Documents Act (Canada) (“PIPEDA”) and comparable legislation at the provincial level, governs the treatment of private information held by a corporation. The Office of the Privacy
33
Commissioner of Canada has stated that it considers the personal information of cannabis users to be considered sensitive. Canadian privacy jurisprudence regarding the obligations that private sector organizations have to individual data subjects is constantly evolving. Privacy laws in Canada are also changing at the legislative level. On November 17, 2020, the Canadian Federal Government introduced Bill C-11, An Act to enact the Consumer Privacy Protection Act and the Personal Information and Data Protection Tribunal and to make consequential amendments to other Acts, for consideration in the House of Commons. Should Bill C-11 come into force, all private organizations that collect, use, and disclose personal information will become subject to new obligations and restrictions, including, without limitation, in connection with obtaining consent, access and control over personal information, deletion of personal information, data portability, de-identification of personal information, and transparency requirements. The penalties and enforcement measures available to Canadian regulators for non-compliance that are contemplated under Bill C-11 and Bill-64 are more significant than those that are available under current privacy and data protection legislation in Canada.
In addition, with respect to consumer health information, there are a number of federal, state and provincial laws protecting the confidentiality of certain patient health information, including patient records, and restricting the use and disclosure of that protected information. For example, the privacy rules under PIPEDA and other applicable privacy laws protect medical records and other personal health information by limiting their use and disclosure of health information to the minimum level reasonably necessary to accomplish the intended purpose and may apply to its operations globally. In Canada, we may also be required to retain certain customer personal information for prescribed periods of time pursuant to the Cannabis Act.
Overall, failure to maintain adequate compliance or safeguards regarding stakeholder privacy could materially adversely impact the Company’s business and result in fines, litigation, or certain government mandated actions, and could be detrimental to the Company’s ability to operate its business. See the section titled “The Company is or may become subject to stringent and evolving U.S. and foreign laws, regulations, rules, contractual obligations, policies and other obligations related to data privacy and security. Its actual or perceived failure to comply with such obligations could lead to regulatory investigations or actions; litigation; fines and penalties; disruptions of the Company's business operations; reputational harm; loss of revenue or profits; and other adverse business consequences” for additional information regarding the privacy laws and regulations to which we may become subject and about the risks to our business associated with such laws and regulations.
The Company’s products may be subject to product recalls or returns, which may result in expense, legal proceedings, regulatory action, loss of sales and reputation, and management attention.
The Companyʼs products may be subject to recall or return for a variety of reasons, including product defects such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labeling disclosure. In certain circumstances, state regulators may change certain regulations after products are already in the market, that may require the Company to issue a recall. If any of the Companyʼs products are recalled due to an alleged product defect or for any other reason, the Company could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection therewith. The Company may lose a significant amount of sales and may not be able to replace those sales at an acceptable margin or at all, in addition to writing off a substantial amount of inventory or materials. Additionally, a product recall may require significant management attention. Although the Company has detailed procedures in place for testing its products, there can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. If one of the products produced by the Company were subject to recall, the image of that product and the Company could be harmed. A recall for any of the foregoing reasons could lead to decreased demand for the Companyʼs products and could have a material adverse effect on the results of operations and financial condition of the Company. Additionally, product recalls may lead to increased scrutiny of the Companyʼs operations by regulatory agencies, requiring further management attention and potential legal fees and other expenses.
The Company faces an inherent risk of product liability claims and other consumer protection claims as a manufacturer, processor and producer of products that are meant to be ingested by people, and dealing with such claims could cause the Company to incur substantial expenses and have a material adverse effect on its business.
As a manufacturer of products designed to be ingested by humans, the Company faces an inherent risk of exposure to product liability claims, regulatory action and litigation if its products are alleged to have caused loss or injury. In addition, the manufacturing and sale of cannabis and other products involve the risk of injury to consumers due to tampering by unauthorized third parties, product contamination, or unauthorized sale of counterfeit products. Previously unknown adverse reactions resulting from human consumption of cannabis products alone or in combination with other medications or substances could occur. Furthermore, safety, efficacy and quality of cannabis in general, or the Company’s products specifically, or associating the consumption of cannabis with illness or other negative effects or events, could have such a material adverse effect on the Company. The Company may be also subject to various product liability claims, including, among others, that the products produced by the Company caused injury or illness, include inadequate instructions for use or include inadequate warnings
34
concerning possible inebriating effects, side effects or interactions with other substances. Consumers may unlawfully operate a vehicle or heavy equipment while under the effects of the Company’s products, resulting in increased liability to the Company. A product liability claim or regulatory action against the Company could result in increased costs, could adversely affect the Companyʼs reputation with its clients and consumers generally, and could have a material adverse effect on the results of operations and financial condition of the Company.
The Companyʼs products may be considered misbranded or adulterated, or otherwise unlawful under federal and state food and drug laws and could subject the Company to local, federal, or state enforcement or private litigation. Some states permit advertising, labeling laws, false and deceptive trade practices, and other consumer-protection laws to be enforced by state attorney generals, who may seek relief for consumers, class action certifications, class wide damages and product recalls of products sold by the Company. Private litigation may also seek relief for consumers, class action certifications, class wide damages and product recalls of products sold by the Company in any of the markets in which it operates. Any actions against the Company by governmental authorities or private litigants could have a material adverse effect on the Companyʼs business, financial condition and results of operations.
The Company may be subject to constraints on and differences in marketing its products under varying regulatory restrictions.
The development of the Companyʼs business and results of operations may be hindered by applicable regulatory restrictions on sales and marketing activities. For example, regulations may prohibit certain sales and marketing activities that may be deemed to target minors or make specified health claims. If the Company is unable to effectively market its products and compete for market share, or if the costs of compliance with government legislation and regulation cannot be absorbed through increased selling prices for the Companyʼs products, the Companyʼs sales and results of operations could be adversely affected.
The Company may be subject to heightened scrutiny by Canadian regulatory authorities, which could negatively affect its business.
The Companyʼs future investments, joint ventures and operations in the United States may become the subject of heightened scrutiny by regulators, stock exchanges and other authorities in Canada. As a result, the Company may be subject to significant direct and indirect interaction with public officials. There can be no assurance that this heightened scrutiny will not in turn lead to the imposition of certain restrictions on the Companyʼs ability to invest in the United States or any other jurisdiction, in addition to those described herein.
Although a memorandum of understanding signed by the Canadian Depository for Securities (“CDS”) and the Canadian recognized exchanges (Aequitas NEO Exchange Inc., the CSE, the Toronto Stock Exchange ("TSX") and the TSX Venture Exchange) dated February 8, 2018, confirms that CDS relies on the exchanges to review the conduct of listed issuers, and therefore there is currently no CDS ban on the clearing of securities of issuers with cannabis-related activities in the United States, there can be no guarantee that this approach to regulation will continue in the future. If such a ban were to be implemented, it would have a material adverse effect on the ability of holders of Common Shares to make and settle trades. In particular, Common Shares would become highly illiquid as and until an alternative was implemented, investors would have no ability to affect a trade of Common Shares through the facilities of a stock exchange.
The Company’s investors and directors, officers and employees who are not U.S. citizens may be denied entry into the U.S., which may negatively affect the Company’s business.
Because cannabis remains illegal under U.S. federal law, those employed at or investing in Canadian or state regulated cannabis companies could face detention, denial of entry or lifetime bans from the United States for their business associations or investments. Entry happens at the sole discretion of the U.S. Customs and Border Protection officers on duty, and these officers have wide latitude to ask questions to determine the admissibility of a foreign national. The Government of Canada has started warning travelers on its website that previous use of cannabis, or any substance prohibited by U.S. federal laws, could mean denial of entry to the U.S. Business or financial involvement in the legal cannabis industry in Canada or in the United States could also be reason enough for U.S. border guards to deny entry.
Because the Company’s contracts involve cannabis and related activities, which are not legal under U.S. federal law, the Company may face difficulties in enforcing its contracts.
It is a fundamental principle of law that a contract will not be enforced if it involves a violation of law or public policy. Because cannabis remains illegal at a federal level, judges may refuse to enforce contracts in connection with activities that violate U.S. federal law, even if there is no violation of state law. There remains doubt and uncertainty that the Company will be able to legally enforce contracts it enters into if necessary. The Company cannot be assured that it will have a remedy for breach of
35
contract, the lack of which may have a material adverse effect on the Companyʼs business, revenues, operating results, financial condition or prospects.
The Company may encounter increasingly strict environmental health and safety regulations in connection with its operations, which may harm the Company’s business.
The Companyʼs operations are subject to environmental and safety laws and regulations concerning, among other things, emissions and discharges to water, air and land, the handling and disposal of hazardous and non-hazardous materials and wastes, and employee health and safety. Changes in environmental, employee health and safety or other laws, more vigorous enforcement thereof or other unanticipated events could require extensive changes to the Companyʼs operations or give rise to material liabilities, which could have a material adverse effect on the business, results of operations and financial condition of the Company.
Risks Related to the Company’s Business, Operations and Industry
The Company’s outstanding indebtedness may adversely affect its business, results of operations and financial condition. the Company’s failure to comply with applicable covenants could trigger events that may materially adversely affect the Company’s business, results of operations and financial condition.
The Company’s outstanding indebtedness may adversely affect the Company’s business, results of operations and financial condition. As of December 31, 2023, the Company had approximately $206,453 of total debt principal amounts outstanding. See the section titled "Item 7, Management's Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources" of this Annual Report for more information regarding the Company's indebtedness. As a result of its indebtedness, a portion of the Company's cash flow will be required to pay interest and principal on its outstanding loans. The Company's indebtedness could have important consequences. For example, it could
The Company expects to use cash flow from operations and outside financings to meet its current and future financial obligations, including funding its operations, debt service and capital expenditures. The Company’s ability to make these payments depends on its future performance, which will be affected by financial, business, economic and other factors, many of which the Company cannot control. The Company’s business may not generate sufficient cash flow from operations in the future, which could result in the Company being unable to repay indebtedness, or to fund other liquidity needs. If the Company does not generate sufficient cash from operations, it may be forced to reduce or delay its business activities and capital expenditures, sell assets, obtain additional debt or equity capital or restructure or refinance all or a portion of its debt on or before maturity. The Company cannot make any assurances that it will be able to accomplish any of these alternatives on terms acceptable to it, or at all. In addition, the terms of existing or future indebtedness may limit the Company’s ability to pursue any of these alternatives.
Furthermore, the instruments governing the Company’s indebtedness include obligations and covenants that limit the Company’s discretion with respect to certain business matters and require the Company to satisfy certain financial requirements. The Company may not receive consent from lenders to take certain actions such as divest assets or enter into material agreements. The Company can make no assurances that it will be able to comply with such obligations and covenants and any failure to comply could result in a default, which, if not amended, cured or waived, could permit acceleration of the indebtedness and the exercise of other remedies available to lenders. In such event, there can be no assurance that the Company would be able to obtain any amendment, cure, waiver or other relief on terms acceptable to the Company. If the Company is
36
unable to obtain relief from an event of the relevant indebtedness may be accelerated and the related collateral may be foreclosed upon, in addition such circumstances may result in the cross-default or cross-acceleration of other debt, any of which would materially adversely affect the Company's business, results of operations, and financial condition.
The Company may require substantial additional financing to operate its business and it may face difficulties acquiring additional financing on terms acceptable to the Company, or at all.
The building and operation of the Companyʼs business, including its facilities, are capital intensive. In order to execute the anticipated growth strategy, the Company may require additional equity and/or debt financing to support on-going operations, to undertake capital expenditures or to undertake acquisitions or other business combination transactions. There can be no assurance that additional financing will be available to the Company when needed or on terms which are acceptable. The Companyʼs inability to raise financing to support on-going operations or to fund capital expenditures or acquisitions could limit the Companyʼs growth and may have a material adverse effect upon future profitability and solvency. Any debt financing secured in the future could involve restrictive covenants relating to capital raising activities and other financial and operational matters, which may make it more difficult for the Company to obtain additional capital and to pursue business opportunities, including potential acquisitions.
Raising additional funds by issuing equity securities will cause dilution to existing stockholders. Raising additional funds through debt financings may involve restrictive covenants and raising funds through lending and licensing arrangements may restrict its operations or require it to relinquish proprietary rights. The Company may not be able to secure additional debt or equity financing on favorable terms or at all.
The Company expects that significant additional capital may be needed in the future to continue its planned operations. The Company expects to finance its cash needs through cash flow from ongoing operations and a combination of equity offerings, debt financings and other strategies. The Company cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to the Company, if at all. If the Company raises additional equity financing, its shareholders may experience significant dilution of their ownership interests, the terms of these securities may include liquidation or other preferences that could adversely affect the rights of a common shareholder, and the per-share value of the Company’s Common Shares could decline. If the Company engages in debt financing, it may be required to accept terms that restrict or limit the Company’s ability to take specific actions, such as incurring additional indebtedness, making capital expenditures or declaring dividends, and other restrictive covenants that could adversely impact the Company’s ability to conduct its business. In addition, weakness and volatility in the capital markets and the economy in general could limit the Company's access to the capital markets and increase its cost of borrowing.
An adverse change in market conditions, including a sustained decline in the price of the Common Shares, negative changes to the Company’s position in the market, or lack of growth in demand for its products and services could be considered to be an impairment triggering event. Such changes could impact valuation assumptions relating to the recoverability of assets and have resulted in, and may in the future result in, impairment charges to the Company’s goodwill or long-lived asset balances, which would negatively impact the Company’s operating results and harm its business.
There are inherent uncertainties in management’s estimates, judgments and assumptions used in assessing recoverability of goodwill, intangible, and other long-lived assets. Any material changes in key assumptions, including failure to meet business plans, a deterioration in the United States, Canadian and global financial markets, an increase in interest rate or an increase in the cost of equity financing by market participants within the industry or other unanticipated events and circumstances, could potentially result in an impairment charge. From time to time, the Company may be required to record a significant charge to earnings in its consolidated financial statements during the period in which any impairment of the Company’s goodwill or intangible and other long-lived assets is determined, which might have a materially adverse impact on the Company’s business operations and its financial position or results of operations.
The Company may be required to write down intangible assets, including goodwill, due to impairment, which could have a material adverse effect on the Company's results of operations or financial position.
The Company may be required to write down intangible assets, including goodwill, due to impairment, which would reduce earnings. The Company periodically calculates the fair value of its reporting units and intangible assets to test for impairment. This calculation may be affected by several factors, including general economic conditions, regulatory developments, market and economic conditions, impact on operations due to changing consumer preferences, successful execution of product launches and overall competitive activity within the market. Certain events can also trigger an immediate review of goodwill and intangible assets. If the carrying value of its reporting unit and other intangible assets exceed their fair value and the loss in value is other than temporary, the goodwill and other intangible assets are considered impaired, which would result in impairment losses and could have a material adverse effect on the Company's consolidated financial position or results of
37
operations. We may be required to perform a quantitative goodwill impairment assessment in future periods for the Company, to the extent the Company experiences declines in the price of its Common Shares.
The Company faces intense competition and its business could be adversely affected by other businesses in a better competitive position.
The introduction of an adult-use model for cannabis production and distribution may impact the growth in the existing medical cannabis market. The impact of this potential development may be negative for the Company and could result in increased levels of competition in its existing medical market and/or the entry of new competitors in the overall cannabis market in which the Company operates, in addition to potential gross margin reduction. There is potential that the Company will face intense competition from other companies, particularly larger multi-state operators, some of which can be expected to have longer operating histories and more financial resources and manufacturing and marketing experience than the Company. Increased competition by larger and better financed competitors could materially and adversely affect the business, financial condition and results of operations of the Company and result in lower than anticipated growth in both the existing medical cannabis market and the adult-use market.
If the number of users of medical cannabis in North America increases, the demand for products will increase and the Company expects that competition will become more intense, as current and future competitors begin to offer an increasing number of diversified products. To remain competitive, the Company may require a continued high level of investment in research and development, marketing, sales and client support, or reduction in product prices. The Company may not have sufficient resources to maintain research and development, marketing, sales and client support efforts on a competitive basis, which could materially and adversely affect the business, financial condition and results of operations of the Company.
Consolidation in the cannabis industry and other changes to the competitive environment can impact the Company's margins and profitability.
The markets for cannabis in the United States and Canada are becoming increasingly competitive and are evolving rapidly. The Company may face intense and increasing competition from existing operators and new entrants in each of the markets in which the Company operates. Some of these competitors may have longer operating histories or offer competitive products. There is also the potential that the cannabis industry will undergo further consolidation that may pose a risk to the Company’s ability to compete.
As a result of this competition, the Company may be unable to maintain its operations or develop them as currently intended. Increased competition by larger, better-financed competitors with geographic advantages could materially and adversely affect its business, financial condition and results of operations. If the Company is unable to achieve its business objectives, such failure could materially adversely affect the Company's business.
As the emerging cannabis industry continues to rapidly evolve, the Company may not be able to create and maintain a competitive advantage in the marketplace. The Company's success will depend on its ability to respond to, among other things, changes in consumer preferences, regulatory conditions, and general competitive pressures. Should the Company be unable to adequately respond to such changes, it could have a material adverse effect on the Company's business, financial condition, operating results, liquidity, cash flow and operational performance.
In each market the Company operates in, the number of licenses granted, and the number of license holders ultimately authorized by the regulator, or previously authorized licenses becoming more commercially active, could have an impact on its business. If the number of users of medical and/or adult-use cannabis increases, the demand for products will increase and the Company expects that competition will become more intense, as current and future competitors begin to offer an increasing number of diversified products. To remain competitive, the Company may require increased levels in research and development, sales and customer support. The Company may not have sufficient resources to maintain research and development, sales and customer support efforts on a competitive basis which could have a material adverse effect on the Company's business, financial condition and results of operations.
The cannabis industry and market are relatively new, and this industry and market may not continue to exist or grow as expected.
The Company is operating its business in a relatively new industry and market. Competitive conditions, consumer preferences, patient requirements and spending patterns in this new industry and market are relatively unknown and may have unique circumstances that differ from existing industries and markets. Accordingly, there are no assurances that this industry and market will continue to exist or grow as currently estimated or anticipated, or function and evolve in a manner consistent with
38
management’s expectations and assumptions. Any event or circumstance that affects the cannabis industry and market could have a material adverse effect on the Companyʼs business, financial condition and results of operations.
The Companyʼs success in North America is dependent on the market building out direct to consumer channels including but not limited to retail outlets. There are many factors which could impact the Companyʼs ability to gain market share and distribute its products, including but not limited to the continued growth and expansion of retail outlets in the North American market which may have a material adverse effect on the Companyʼs business, operating results and financial condition. The Companyʼs ability to continue to grow, process, store and sell medical cannabis and participate in the adult-use cannabis markets is dependent on the maintenance and validity of the Companyʼs licenses from regulatory authorities.
The Company's profitability may be impacted by declining wholesale or retail cannabis prices in certain markets and shifting market conditions.
The Company’s gross profits may decline as a result of reduction in wholesale or retail cannabis prices. The Company’s profitability is sensitive to fluctuations in wholesale and retail prices caused by crop seasonality, disruptions to supply chains, increased competition, government taxes or levies, and other market conditions, all of which are factors beyond the Company’s control. There is currently not an established market price for cannabis and the price of may change rapidly. Any price decline may have a material adverse effect on the Company.
If a significant number of new licenses are granted by a regulator in a market in which the Company operates, the Company may experience increased competition for market share and may experience downward price pressure on its products as new entrants increase production. The Company may also face competition from illegal cannabis dispensaries that are selling cannabis to individuals despite not having a valid license. The continued declining wholesale and retail price will impact the Company’s overall business.
The Company has historically had negative cash flow from operating activities, and continued losses could have a material negative effect on The Company's business and prospects and impact its ability to continue as a going concern.
The Company started sales in April 2018 and historically has had negative cash flow from operating activities. As of December 31, 2023, the Company had an accumulated deficit of approximately $704,162. The Company incurred losses in fiscal year 2022 and fiscal year 2023. The Company may not be able to achieve or maintain profitability and may continue to incur significant losses in the future. The Company's cash flow and net losses for the year ended December 31, 2023 are indicators that raise substantial doubt about the Company's ability to continue as a going concern for at least one year from the issuance of these financial statements. In addition, debt service requirements will have an adverse impact on liquidity. Because the cannabis industry and market are relatively new and rapidly evolving, it is difficult for the Company to predict its future operating results. As a result, it may incur future losses that may be larger than anticipated. In addition, the Company may continue to increase operating expenses as it implements initiatives to continue to grow its business. The Company's ability to execute on its growth strategy requires significant additional financing. The Company may need to raise additional funds in order to operate its business and meet obligations as they become due. However, financing may not be available to the Company in the necessary time frame, in amounts that the Company requires, on terms that are acceptable to the Company, or at all. If the Company is unable to raise the necessary funds when needed, it will be required to take additional actions to address its liquidity needs, including cost reduction measures, it would materially and adversely impact the Company's ability to execute on its operating plans. If the Company becomes unable to continue as a going concern, the Company may have to dispose of assets and might realize significantly less than the values at which are carried on its consolidated financial statements. These actions may cause the Company's stockholders to lose all or part of their investment in the Company's Common Stock. If the Companyʼs sales do not increase to offset these expected increases in costs and operating expenses, the Company will not be profitable. Furthermore, if the Company's future growth and operating performance fail to meet investor or analyst expectations, or if it has future negative cash flow or losses resulting from its investment in product development or marketing, its financial condition and share price could be materially adversely affected.
The Company may be affected by currency fluctuations.
The Company may face exposure to currency fluctuations because of its present operations in the United States. Substantially all of the Company’s revenue is earned in U.S. dollars, but its shares, and some of its employee stock options and certain warrants issued to holders of the Company’s notes are denominated in Canadian dollars which can provide variability for results of operations. The Company does not have currency hedging arrangements in place and there is no expectation that it will put
39
any currency hedging arrangements in place in the future. Fluctuations in the exchange rate between the U.S. dollar and the Canadian dollar may have a material adverse effect on the Company’s business, financial position or results of operations.
Demand for the Company’s products is difficult to forecast due to limited and unreliable market data.
As a result of recent and ongoing regulatory and policy changes in the medical and adult-use cannabis industry, the market data available is limited and unreliable. Federal and state laws prevent widespread participation and hinder market research. Therefore, the Company must rely largely on its own market research to forecast sales as detailed forecasts are not generally obtainable from other sources at this early stage of the industry. Market research and projections by the Company of estimated total retail sales, demographics, demand, and similar consumer research are based on assumptions from limited and unreliable market data, and generally represent the personal opinions of the Companyʼs management team as of the date of this Annual. A failure in the demand for its products to materialize as a result of competition, technological change or other factors could have a material adverse effect on the business, results of operations, financial condition or prospects of the Company.
The Company’s inability to attract and retain key personnel, or its inability to maintain relations with its employees, unions and other employee representatives, could materially adversely affect its business.
The success of the Company is dependent upon the ability, expertise, judgment, discretion and good faith of its senior management, which are key personnel. Moreover, the Companyʼs future success depends on its continuing ability to attract, develop, motivate and retain highly qualified and skilled employees. Qualified individuals are in high demand, and the Company may incur significant costs to attract and retain them. The Company does not maintain "key man" insurance policies on the lives of its key personnel, or the lives of any of its other employees. In order to induce valuable employees to continue their employment with the company, the Company has provided certain key personnel stock options and restricted stock units that vest over time. The value to employees of such equity grants that vest over time is significantly affected by movements in the Company's share price that are beyond the Company's control, and may at any time be insufficient to counteract more lucrative offers from other companies. In addition, if the Company's share-based compensation otherwise ceases to be viewed as a valuable benefit, the Company's ability to attract, retain and motivate key personnel could be weakened, which could harm its business. The loss of the services of key personnel, or an inability to attract other suitably qualified persons when needed, could have a material adverse effect on the Companyʼs ability to execute on its business plan and strategy, and the Company may be unable to find adequate replacements on a timely basis, or at all. While employment agreements are customarily used as a primary method of retaining the services of such key personnel these agreements cannot assure the continued services of such employees.
There is no assurance that any of the Companyʼs existing personnel who presently or may in the future require a security clearance will be able to obtain or renew such clearances or that new personnel who require a security clearance will be able to obtain one. A failure by such key personnel to maintain or renew their security clearance would result in a material adverse effect on the Companyʼs business, financial condition and results of operations. In addition, if any key personnel leave the Company, and the Company is unable to find a suitable replacement that has a security clearance in a timely manner, or at all, it could have a material adverse effect on the Companyʼs business, financial condition and results of operations.
The Company may not be able to attract or retain qualified management and other key employees in the future due to the intense competition for a limited number of qualified personnel in its industry. Many of the other cannabis companies that it competes against for qualified personnel have greater financial and other resources, different risk profiles and a longer history in the industry than the Company does. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high quality candidates than what the Company has to offer. If the Company is unable to continue to attract and retain high quality personnel, the rate and success at which it can develop and market its products will be limited.
In addition, certain of the Company’s employees are covered by collective bargaining agreements. These agreements typically contain provisions regarding the general working conditions of such employees, including provisions that could affect the Company’s ability to restructure its operations, close facilities, or reduce the number of its employees. The Company may not be able to extend existing collective bargaining agreements or, upon the expiration of such agreements, negotiate such agreements in a favorable and timely manner or without work stoppages, strikes or similar actions. Any deterioration of the relationships with its employees, unions and other employee representatives, or any material work stoppage, strike or similar action could have a material adverse effect on the Company’s business results, cash flows, financial condition or prospects. Furthermore, The Company’s actions or responses to any such negotiations, labor disputes, work stoppages or strikes could negatively impact its corporate reputation and have adverse effects on its business.
40
The Company may face unfavorable publicity or consumer perception of the safety, efficacy and quality of its cannabis products.
The Company believes the cannabis industry is highly dependent upon consumer perception regarding the safety, efficacy and quality of the cannabis distributed to such consumers. Consumer perception of the Companyʼs products can be significantly influenced by scientific research or findings, regulatory investigations, litigation, media attention and other publicity regarding the consumption of medical cannabis products. There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favorable to the medical cannabis market or any particular product, or consistent with earlier publicity.
Future research reports, findings, regulatory proceedings, litigation, media attention or other publicity that are perceived as less favorable than, or that question, earlier research reports, findings or publicity could have a material adverse effect on the demand for the Companyʼs products and the business, results of operations, financial condition of the Company. In particular, adverse publicity reports or other media attention regarding the safety, efficacy and quality of cannabis in general, or the Companyʼs products specifically, or associating the consumption of cannabis with illness or other negative effects or events, could have such a material adverse effect. Such adverse publicity reports or other media attention could arise even if the adverse effects associated with such products resulted from consumers’ failure to consume such products appropriately or as directed.
Further, adverse publicity reports or other media attention regarding the safety, efficacy and quality of cannabis in general, or the Company’s products specifically, or associating the consumption of cannabis with illness or other negative effects or events, could have such a material adverse effect on the Company. Although the Company uses quality control processes and procedures to ensure our consumer packaged goods meet our standards, a failure or alleged failure of such processes and procedures could result in negative consumer perception of products or legal claims against the Company. Adverse publicity reports or other media attention could arise even if the adverse effects associated with such products resulted from consumers’ failure to consume such products appropriately or as directed.
The Company offers certain vaporizer or “vape” products. The use of vape products and vaping may pose health risks. According to the Centers for Disease Control, vape products may contain ingredients that are known to be toxic to humans and may contain other ingredients that may not be safe. Because clinical studies about the safety and efficacy of vape products have not been submitted to the FDA, consumers currently have no way of knowing whether they are safe for their intended use or what types or concentrations of potentially harmful chemicals or by-products are found in these products. It is also uncertain what implications the use of vape or other inhaled products, such as cannabis flower that is smoked, may have on respiratory illnesses. Although the Company believes that it takes care in protecting its image and reputation, the Company does not ultimately have direct control over how it is perceived by others. Adverse findings, regulatory investigations, litigation, media attention and other publicity regarding the consumption of vape or other inhaled products, including adverse publicity regarding underage use of vape or other inhaled products, may result in reputation loss and in decreased investor confidence, increased challenges in developing and maintaining community relations and an impediment to the Companyʼs overall ability to advance its business, thereby having a material adverse impact on the financial condition and results of operations of the Company.
The Company faces reputational risks, which may negatively impact its business.
Damage to the Companyʼs reputation can be the result of the actual or perceived occurrence of any number of events, and could include any negative publicity, whether true or not. The increased usage of social media and other web-based tools used to generate, publish, and discuss user-generated content and to connect with other users has made it increasingly easier for individuals and groups to communicate and share opinions and views regarding the Company and its activities, whether true or not. Although the Company believes that it operates in a manner that is respectful to all shareholders and that it takes care in protecting its image and reputation, the Company does not ultimately have direct control over how it is perceived by others. Reputation loss may result in decreased investor confidence, increased challenges in developing and maintaining community relations, and an impediment to the Companyʼs overall ability to advance its projects, thereby having a material adverse impact on financial performance, financial condition, cash flows, and growth prospects. Further, the parties with which the Company does business may perceive that they are exposed to reputational risk as a result of the Companyʼs cannabis business activities. Failure to establish or maintain business relationships could have a material adverse effect on the Company.
The Company is dependent on suppliers and key inputs for the cultivation, extraction and production of cannabis products.
The ability of the Company to compete and grow will be dependent on it having access, at a reasonable cost and in a timely manner, to required equipment, parts and components. No assurances can be given that the Company will be successful in maintaining its required supply of equipment, parts and components. Global supply challenges have added further uncertainties around availability and price of equipment, parts and components. It is also possible that the final costs of the major equipment contemplated by the Companyʼs capital expenditure plans may be significantly greater than anticipated by the Companyʼs
41
management and may be greater than funds available to the Company, in which circumstance the Company may curtail, or extend the timeframes for completing, its capital expenditure plans. This could have an adverse effect on the business, financial condition, results of operations or prospects of the Company.
The cannabis business is dependent on a number of key inputs and their related costs including raw materials and supplies related to growing operations, as well as electricity, water and other local utilities. Any significant interruption or negative change in the availability or economics of the supply chain for key inputs could materially impact the business, financial condition, results of operations or prospects of the Company. In addition, any restrictions on the ability to secure required supplies or utility services or to do so on commercially acceptable terms could have a materially adverse impact on the business, financial condition and operating results. Some of these inputs may only be available from a single supplier or a limited group of suppliers. If a sole source supplier was to go out of business, the Company might be unable to find a replacement for such source in a timely manner or at all. If a sole source supplier were to be acquired by a competitor, that competitor may elect not to sell to the Company in the future. Any inability to secure required supplies and services or to do so on appropriate terms and/or agreeable terms could have a materially adverse impact on the business, financial condition, results of operations or prospects of the Company.
The Companyʼs business is subject to the risks inherent in agricultural operations.
The Companyʼs business involves the cultivation of the cannabis plant. The cultivation of this plant is subject to agricultural risks related to insects, plant diseases, unstable growing conditions, water and electricity availability and cost, and force majeure events. Certain agricultural risks, such as insects or plant diseases, can result in a prolonged disruption of its operations. Although the Company cultivates its cannabis plants indoors or in greenhouses, each with climate-controlled rooms staffed by trained personnel, there can be no assurance that agricultural risks will not have a material adverse effect on the cultivation of its cannabis. The Company may in the future cultivate cannabis plants outdoors, which would also subject it to related agricultural risks.
The Company may be adversely impacted by rising or volatile energy costs.
The Companyʼs cannabis growing and manufacturing operations consume considerable energy, which make the Company vulnerable to rising energy costs. Accordingly, rising or volatile energy costs have and may further adversely impact the Company's business and profitability. Furthermore, the Company may face challenges in accessing sufficient utilities to meet the energy, water or sewage requirements for cannabis growing and manufacturing operations.
The Companyʼs intellectual property may be difficult to protect, and failure to do so may negatively impact its business.
The ownership and protection of trademarks, patents, trade secrets and intellectual property rights are significant aspects of the Companyʼs future success. The Company has no patented technology or trademarked business methods at this time, nor has it registered any patents. The Company has filed trademark applications in the United States and Canada. The Company will continue to seek trademark protection in the United States and Canada.
The Company’s ability to obtain registered trademark protection for cannabis-related goods and services, in particular for cannabis itself, may be limited in certain countries outside of Canada, including the United States, where registered federal trademark protection is currently unavailable for trademarks covering cannabis-related products and services that are illegal under the Controlled Substances Act. Accordingly, the Company’s ability to obtain intellectual property rights or enforce intellectual property rights against third party uses of similar trademarks may be limited in certain countries. The U.S. Patent and Trademark Office released a policy on May 2, 2019 that clarifies that applications for trademarks for products that meet the definition of hemp could be accepted for registration, with certain exceptions.
Even if the Company moves to protect its technology with trademarks, patents, copyrights or by other means, the Company is not assured that competitors will not develop similar technology, business methods or that the Company will be able to exercise its legal rights. The Company may have limited or no enforcement options should its intellectual property be infringed on by illicit operators. Furthermore, other countries may not protect intellectual property rights to the same standards as the United States or Canada. Actions taken to protect or preserve intellectual property rights may require significant financial and other resources which may have a significant impact on the Companyʼs ability to successfully grow the business.
In addition, other parties may claim that the Companyʼs products infringe on their proprietary and perhaps patent protected rights. Such claims, whether or not meritorious, may result in the Companyʼs expenditure of significant financial and managerial resources, legal fees, result in injunctions, temporary restraining orders and/or require the payment of damages.
The Company and investors may have difficulty enforcing their legal rights.
42
In the event of a dispute arising from TerrAscendʼs U.S. operations, TerrAscend may be subject to the exclusive jurisdiction of foreign courts or may not be successful in subjecting foreign persons to the jurisdictions of courts in Canada. Similarly, to the extent that the Companyʼs assets are located outside of Canada, investors may have difficulty collecting from the Company any judgments obtained in the Canadian courts and predicated on the civil liability portions of securities provisions. The Company may also be hindered or prevented from enforcing its rights with respect to a governmental entity or instrumentality because of the doctrine of sovereign immunity.
The Company (and the third parties upon which it relies) faces physical security risks, as well as risks related to its information technology systems, potential cyber-attacks, and security breaches.
In the ordinary course of its business, the Company, and the third parties upon which it relies, collects, receives, stores, processes, generates, uses, transfers, discloses, makes accessible, protects, secures, disposes of, transmits, and shares personal data about its patients, adult-use customers and guests and other sensitive information, including proprietary and confidential business data, trade secrets, intellectual property, sensitive third-party data, business plans, transactions, and financial information (collectively, "sensitive data"). The Company is responsible for protecting sensitive data from security breaches.
If there was a breach in physical security systems and the Company became victim to a robbery or theft or if there was a failure of information technology systems or a component of information technology systems, or if the Company's sensitive data were compromised, it could, depending on the nature of any such breach, failure or compromise, adversely impact the Companyʼs reputation, business continuity and results of operations. Any such security breach, failure or compromise could expose the Company to additional liability and to potentially costly litigation, government enforcement actions, additional reporting requirements and/or oversight, indemnification obligations, negative publicity, increase expenses relating to the resolution and future prevention of these breaches and may deter potential customers from choosing the Companyʼs products. Given the nature of the Companyʼs products and its lack of legal availability outside of channels approved by the government of the United States, as well as the concentration of inventory in its facilities, there remains a risk of shrinkage as well as theft.
A security breach may occur through procedural or process failure, information technology malfunction, or deliberate unauthorized intrusions. Cyber-attacks, malicious internet-based activity, online and offline fraud, and other similar activities threaten the confidentiality, integrity, and availability of the Company’s sensitive data and information technology systems, and those of the third parties upon which it relies. Such threats are prevalent and continue to rise, are increasingly difficult to detect, and come from a variety of sources, including traditional computer “hackers,” threat actors, “hacktivists,” organized criminal threat actors, personnel (such as through theft or misuse), sophisticated nation states, and nation-state-supported actors.
Some actors now engage and are expected to continue to engage in cyber-attacks, including without limitation nation-state actors for geopolitical reasons and in conjunction with military conflicts and defense activities. During times of war and other major conflicts, the Company and the third parties upon which it relies may be vulnerable to a heightened risk of these attacks, including retaliatory cyber-attacks, that could materially disrupt its systems and operations, supply chain, and ability to produce, sell and distribute its services.
The Company and the third parties upon which it relies are subject to a variety of evolving threats, including but not limited to social-engineering attacks (including through phishing attacks), malicious code (such as viruses and worms), malware (including as a result of advanced persistent threat intrusions), denial-of-service attacks (such as credential stuffing), credential harvesting, personnel misconduct or error, ransomware attacks, supply-chain attacks, software bugs, server malfunctions, software or hardware failures, loss of data or other information technology assets, adware, telecommunications failures, earthquakes, fires, floods, cable cuts, damage to physical plants, power loss, vandalism, theft, and other similar threats.
In particular, severe ransomware attacks are becoming increasingly prevalent and can lead to significant interruptions in the Company’s operations, loss of sensitive data and income, reputational harm, and diversion of funds. Extortion payments may alleviate the negative impact of a ransomware attack, but the Company may be unwilling or unable to make such payments due to, for example, applicable laws or regulations prohibiting such payments.
Remote work has become more common and has increased risks to the Company’s information technology systems and data, as more of its employees utilize network connections, computers, and devices outside its premises or network, including working at home, while in transit and in public locations. Additionally, future or past business transactions (such as acquisitions or integrations) could expose the Company to additional cybersecurity risks and vulnerabilities, as its systems could be negatively affected by vulnerabilities present in acquired or integrated entities’ systems and technologies. Furthermore, the Company may discover security issues that were not found during due diligence of such acquired or integrated entities, and it may be difficult to integrate companies into the Company’s information technology environment and security program should any security issues be identified.
43
In addition, the Company's reliance on third-party service providers could introduce new cybersecurity risks and vulnerabilities, including supply-chain attacks, and other threats to its business operations. The Company has entered into agreements with third parties for hardware, software, telecommunications and other information technology (or “IT”) services in connection with its operations. The Company’s ability to monitor these third parties’ information security practices is limited, and these third parties may not have adequate information security measures in place. If its third-party service providers experience a security incident or other interruption, the Company could experience adverse consequences. While the Company may be entitled to damages if its third-party service providers fail to satisfy their privacy or security-related obligations to the Company, any award may be insufficient to cover the Company’s damages, or the Company may be unable to recover such award. In addition, supply-chain attacks have increased in frequency and severity, and the Company cannot guarantee that third parties’ infrastructure in its supply chain or its third-party partners’ supply chains have not been compromised.
The Companyʼs operations depend, in part, on how well it and its suppliers protect networks, equipment, IT systems and software against damage from of the aforementioned or similar threats. The Companyʼs operations also depend on the timely maintenance, upgrade and replacement of networks, equipment, IT systems and software, as well as pre-emptive expenses to mitigate the risks of failures. Any of these and other events could the Company’s ability to monitor these third parties’ information security practices is limited, and these third parties may not have adequate information security measures in place. If its third-party service providers experience a security incident or other interruption, the Company could experience adverse consequences. While the Company may be entitled to damages if its third-party service providers fail to satisfy their privacy or security-related obligations to the Company, any award may be insufficient to cover the Company’s damages, or the Company may be unable to recover such award. In addition, supply-chain attacks have increased in frequency and severity, and the Company cannot guarantee that third parties’ infrastructure in its supply chain or its third-party partners’ supply chains have not been compromised.
The Company may expend significant resources or modify its business activities to try to protect against security breaches. Additionally, certain data privacy and security obligations may require the Company to implement and maintain specific security measures or industry-standard or reasonable security measures to protect its information technology systems and sensitive data.
While the Company has implemented security measures designed to protect against security breaches, there can be no assurance that these measures will be effective. The Company takes steps to detect and remediate vulnerabilities, but it may not be able to detect and remediate all vulnerabilities because the threats and techniques used to exploit the vulnerability change frequently and are often sophisticated in nature. Therefore, such vulnerabilities could be exploited but may not be detected until after a security incident has occurred. These vulnerabilities pose material risks to the Company’s business. Further, the Company may experience delays in developing and deploying remedial measures designed to address any such identified vulnerabilities.
Applicable data privacy and security obligations may require the Company to notify relevant stakeholders of security breaches. Such disclosures are costly, and the disclosure or the failure to comply with such requirements could lead to adverse consequences.
The Company’s contracts may not contain limitations of liability, and even where they do, there can be no assurance that limitations of liability in its contracts are sufficient to protect it from liabilities, damages, or claims related to its data privacy and security obligations. The Company cannot be sure that its insurance coverage will be adequate or sufficient to protect it from or to mitigate liabilities arising out of its privacy and security practices, that such coverage will continue to be available on commercially reasonable terms or at all, or that such coverage will pay future claims.
In addition to experiencing a security breach, third parties may gather, collect, or infer sensitive data about it from public sources, data brokers, or other means that reveals competitively sensitive details about the Company and could be used to undermine its competitive advantage or market position.
The Company is or may become subject to stringent and evolving U.S. and foreign laws, regulations, rules, contractual obligations, policies and other obligations related to data privacy and security. Its actual or perceived failure to comply with such obligations could lead to regulatory investigations or actions; litigation; fines and penalties; disruptions of the Company's business operations; reputational harm; loss of revenue or profits; and other adverse business consequences.
The Company’s data processing activities may subject it to numerous data privacy and security obligations, such as various laws, regulations, guidance, industry standards, external and internal privacy and security policies, contractual requirements, and other obligations relating to data privacy and security.
In the United States, federal, state, and local governments have enacted numerous data privacy and security laws, including data breach notification laws, personal data privacy laws, consumer protection laws (e.g., Section 5 of the Federal Trade
44
Commission Act), and other similar laws (e.g., wiretapping laws). For example, there are a number of federal, state, and local laws protecting the confidentiality of certain health-related information of the Company’s patients and customers, including their health-related records, and restricting the use and disclosure of that information.
Additionally, in the past few years, numerous U.S. states including California, Virginia, Colorado, Connecticut, and Utah have enacted comprehensive privacy laws that impose certain obligations on covered businesses, including providing specific disclosures in privacy notices and affording residents with certain rights concerning their personal data. As applicable, such rights may include the right to access, correct, or delete certain personal data, and to opt-out of certain data processing activities, such as targeted advertising, profiling, and automated decision-making. The exercise of these rights may impact our business and ability to provide our products and services. Certain states also impose stricter requirements for processing certain personal data, including sensitive information, such as conducting data privacy impact assessments. These state laws allow for statutory fines for noncompliance. For example, the CCPA, with amendments from the California Privacy Rights Act of 2020 (“CPRA”), applies to personal data of consumers, business representatives, and employees who are California residents, and requires businesses to provide specific disclosures in privacy notices and honor requests of such individuals to exercise certain privacy rights. The CCPA provides for fines of up to $7,500 per intentional violation and allows private litigants affected by certain data breaches to recover significant statutory damages.
Similar laws have passed or are being considered in several other states, as well as at the federal and local levels, and we expect more states to pass similar laws in the future. These developments may further complicate compliance efforts, as well as increase legal risk and compliance costs for us and the third parties upon whom we rely.
Furthermore, the Controlling the Assault of Non-Solicited Pornography and Marketing Act of 2003 and the TCPA impose specific requirements on communications with customers. For example, the TCPA imposes various consumer consent requirements and other restrictions on certain telemarketing activity and other communications with consumers by phone, fax or text message. TCPA violations can result in significant financial penalties, including penalties or criminal fines imposed by the Federal Communications Commission or fines of up to $1,500 per violation imposed through private litigation or by state authorities. Outside the United States, an increasing number of laws, regulations, and industry standards may govern data privacy and security. For example, Canada’s PIPEDA imposes strict requirements for processing personal data.
In addition to data privacy and security laws, the Company is bound, or may become bound, by other obligations related to data privacy and security, including contractual obligations and self-regulatory standards (e.g., PCI-DSS) and its efforts to comply with such obligations may not be successful. For example, we rely on vendors to process payment card data who may be subject to PCI-DSS, and our business may be negatively impacted if our vendors are fined or suffer other consequences as a result of PCI-DSS noncompliance. Additionally, the Company publishes privacy policies, marketing materials, and other public or external facing statements. If found to be deficient, lacking in transparency, deceptive, unfair, or misrepresentative of facts or its practices, the Company may be subject to investigation, enforcement actions by regulators, or experience other adverse consequences.
Obligations related to data privacy and security are quickly changing, becoming increasingly stringent, and creating regulatory uncertainty. Additionally, these obligations may be subject to differing applications and interpretations, which may be inconsistent or conflict among jurisdictions. Preparing for and complying with these obligations requires the Company to devote significant resources and may necessitate changes to its services, information technologies, systems, and practices and to those of any third parties that process personal data on its behalf.
The Company may at times fail, or be perceived to have failed, in its efforts to comply with its data privacy and security obligations. Moreover, despite its efforts, the Company’s personnel or third parties on whom it relies may fail to comply with such obligations, which could negatively impact its business operations. If the Company or the third parties on which it relies fail, or are perceived to have failed, to address or comply with applicable data privacy and security obligations, it could face significant consequences, including but not limited to: government enforcement actions (e.g., investigations, fines, penalties, audits, inspections, and similar); litigation (including class-action claims); additional reporting requirements and/or oversight; bans on processing personal data; and orders to destroy or not use personal data. Any of these events could have a material adverse effect on the Company’s reputation, business, or financial condition, including but not limited to: loss of customers; inability to process personal data or to operate in certain jurisdictions; limited ability to develop or commercialize its products; expenditure of time and resources to defend any claim or inquiry; adverse publicity; or substantial changes to its business model or operations.
45
The Company faces exposure to fraudulent or illegal activity by employees, contractors and consultants, which may subject the Company to investigations or other actions.
The Company is exposed to the risk that its employees, independent contractors and consultants may engage in fraudulent or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to the Company that violates: (i) government regulations; (ii) manufacturing standards; (iii) federal, state and provincial healthcare fraud and abuse laws and regulations; or (iv) laws that require the true, complete and accurate reporting of financial information or data. It may not always be possible for the Company to identify and deter misconduct by its employees and other third parties, and the precautions taken by the Company to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting the Company from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against the Company, and it is not successful in defending itself or asserting its rights, those actions could have a significant impact on the Companyʼs business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of the Companyʼs operations, any of which could have a material adverse effect on the Companyʼs business, financial condition, results of operations or prospects.
Directors and officers of the Company have faced, and may in the future face, conflicts of interests regarding the business strategy of the Company.
Certain of the directors and officers of the Company are also directors and officers of other companies or are engaged and will continue to be engaged in activities that may put them in conflict with the business strategy of the Company. Consequently, there exists the possibility for such directors and officers to be in a position of conflict.
In particular, the Company may also become involved in other transactions which conflict with the interests of its directors and officers, who may from time-to-time deal with persons, firms, institutions or companies with which the Company may be dealing, or which may be seeking investments similar to those desired by it. All decisions to be made by directors and officers of the Company are required to be made in accordance with their duties and obligations to act honestly and in good faith with a view to the best interests of the Company. In addition, the directors and officers are required to declare their interests in, and such directors are required to refrain from voting on, any matter in which they may have a material conflict of interest. Failure to adequately manage or disclose conflicts of interest may result in public accusations, investigations, or litigation, and could have material adverse effect on the Company's business, operating results and financial condition.
The Company’s internal controls over financial reporting may not be effective, and the Company’s independent auditors may not be able to certify as to their effectiveness, which could have a significant and adverse effect on the Company’s business.
The Company is subject to the reporting requirements of the Exchange Act and the Sarbanes-Oxley Act of 2002, as amended (the "Sarbanes-Oxley Act"). Pursuant to Section 404 of the Sarbanes-Oxley Act, the Company is required to perform system and process evaluation and testing of its internal control over financial reporting to allow Company management to report on the effectiveness of its internal control over financial reporting. Furthermore, if at such time, the Company no longer qualifies as an "emerging growth company," its independent registered public accounting firm will be required to issue an annual report that attests to the effectiveness of the Company's internal control over financial reporting.
The Company incurs expenses and diversion of Company management’s time in its efforts to comply with Section 404 of the Sarbanes-Oxley Act regarding internal controls over financial reporting. Effective internal controls over financial reporting are necessary for the Company to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause the Company to fail to meet its reporting obligations. In addition, any testing by the Company conducted in connection with Section 404 of the Sarbanes-Oxley Act, or the subsequent testing by the Company’s independent registered public accounting firm when required, may reveal deficiencies in the Company’s internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retrospective changes to the Company’s consolidated financial statements or identify other areas for further attention or improvement. Moreover, the Company's internal controls over financial reporting will not prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system's objective will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud will be detected. If the Company is unable to assert that its internal control over financial reporting is effective, investors could lose confidence in the Company's reported financial information, the trading price of the Company's Common Shares could decline and the Company
46
could be subject to sanctions or investigations by the United States Securities and Exchange Commission or other regulatory authorities.
The Company’s business could be adversely affected by economic downturns, inflation, increases in interest rates, natural disasters, public health crises, political crises, geopolitical events, such as the war in Ukraine and the hostilities in the Middle East, or other macroeconomic conditions, which have in the past and may in the future negatively impact the Company’s business and financial performance.
The global economy, including credit and financial markets, has experienced extreme volatility and disruptions, including, among other things, severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, supply chain shortages, increases in inflation rates, higher interest rates and uncertainty about economic stability. For example, the COVID-19 pandemic resulted in widespread unemployment, economic slowdown and extreme volatility in the capital markets. The U.S. Federal Reserve recently raised interest rates multiple times in response to concerns about inflation and it may raise them again. Higher interest rates, coupled with reduced government spending and volatility in financial markets may increase economic uncertainty and affect consumer spending. If the equity and credit markets deteriorate, including as a result of political unrest or war, such as the war in Ukraine and the hostilities in the Middle East, may make any necessary debt or equity financing more difficult to obtain in a timely manner or on favorable terms, more costly or more dilutive. Increased inflation rates can adversely affect the Company by increasing the Company’s costs, including labor and employee benefit costs.
The Company may not have access to United States bankruptcy protections available to non-cannabis businesses.
Because cannabis is a Schedule I controlled substance under the Controlled Substances Act, courts may deny cannabis businesses federal bankruptcy protections, making it difficult for lenders to be made whole on their investments in the cannabis industry in the event of a bankruptcy. If the Company were to experience a bankruptcy, there is no guarantee that United States federal bankruptcy protections would be available to us, which would have a material adverse effect on us and may make it more difficult for us to obtain debt financing.
The development of the Company’s products is complex and requires significant investment. Failure to develop new methodologies and products could adversely affect the Company’s business.
The introduction of new products embodying new methodologies, including new manufacturing processes, and the emergence of new industry standards may render the Companyʼs products obsolete, less competitive or less marketable. The process of developing the Companyʼs products is complex and requires significant continuing costs, development efforts and third-party commitments. The Companyʼs failure to develop new methodologies and products and the obsolescence of existing methodologies could adversely affect the business, financial condition and operating results of the Company. The Company may be unable to anticipate changes in its potential customer requirements that could make the Companyʼs existing methodologies obsolete.
The development of the Companyʼs proprietary methodologies entails significant technical and business risks. The Company may not be successful in using its new methodologies or exploiting its niche markets effectively or adapting its businesses to evolving customer or medical requirements or preferences or emerging industry standards.
The Company needs to attract and retain customers and patients in order to succeed, and failure to do so may have a material adverse effect on the Company’s business.
The Companyʼs success depends on its ability to attract and retain customers and patients. There are many factors which could impact the Companyʼs ability to attract and retain customers and patients, including but not limited to the Companyʼs ability to continually produce desirable and effective products and, the successful implementation of a customer and patient-acquisition plan. The Companyʼs failure to acquire and retain customers and patients would have a material adverse effect on the Companyʼs business, operating results and financial condition.
The Company has a limited operating history, which makes it difficult to evaluate its prospects and predict future operating results.
The Company has a limited operating history and, accordingly, potential investors will have a limited basis on which to evaluate its ability to achieve its business objectives. The future success of the Company is dependent on management’s ability to implement its strategy, there is no certainty that anticipated outcomes and sustainable revenue streams will be achieved and there is no certainty that the Company will successfully produce commercial cannabis, establish a market for and sell its product, maintain its licenses or obtain other necessary licenses and/or approvals.
47
The Company faces risks frequently encountered by early-stage companies. In particular, its future growth and prospects will depend on its ability to expand its operation and gain additional revenue streams while at the same time maintaining effective cost controls. Any failure to expand is likely to have a material adverse effect on the Companyʼs business, financial condition, and results. As such, there is no assurance that the Company will be successful in achieving a return on the Company shareholders’ investment and the likelihood of success must be considered in light of the early stage of operations.
The Company may be subject to growth-related risks, which could negatively affect its business.
The Company may be subject to growth-related risks including capacity constraints and pressure on its internal systems and controls. The ability of the Company to manage growth effectively will require it to continue to implement and improve its operational and financial systems and to expand, train and manage its employee base. The inability of the Company to deal with this growth may have a material adverse effect on the Companyʼs business, financial condition, results of operations and prospects.
Risks Related to the Company’s Investment and Acquisition Business Strategies
The success of the Companyʼs business depends, in part, on its ability to successfully integrate recently acquired businesses and to retain key employees of acquired businesses. If the Company is unsuccessful in doing so, it may negatively affect the Companyʼs business.
The Company may not be able to successfully integrate and combine the operations, personnel, and technology infrastructure of any such acquired company with its existing operations. If integration is not managed successfully by the Companyʼs management, the Company may experience interruptions to its business activities, deterioration in its employee and customer relationships, increased costs of integration and harm to its reputation, all of which could have a material adverse effect on the Companyʼs business, financial condition, and results of operations. The Company may experience difficulties in combining corporate cultures, maintaining employee morale, and retaining key employees. The integration of any such acquired companies may also impose substantial demands on management. There is no assurance that these acquisitions will be successfully integrated in a timely manner, or at all.
There can be no assurance that the Company’s current and future strategic alliances will have a beneficial impact on the Company’s business, financial condition, and results of operations.
The Company currently has, and may in the future, enter into strategic alliances with third parties it believes will complement or augment its existing business. The Companyʼs ability to complete strategic alliances is dependent upon, and may be limited by, the availability of suitable candidates and capital. In addition, strategic alliances could present unforeseen integration obstacles or costs, may not enhance the Companyʼs business, and may involve risks that could adversely affect the Company, including significant amounts of management time that may be diverted from operations in order to pursue and complete such transactions or maintain such strategic alliances. Future strategic alliances could result in the incurrence of additional debt, costs and contingent liabilities, and there can be no assurance that future strategic alliances will achieve, or that the Companyʼs existing strategic alliances will continue to achieve, the expected benefits to the Companyʼs business or that the Company will be able to consummate future strategic alliances on satisfactory terms, or at all. Any of the foregoing could have a material adverse effect on the Companyʼs business, financial condition and results of operations.
The Company’s use of joint ventures may expose it to risks associated with jointly owned investments.
The Company currently operates parts of its business through joint ventures with other companies, and it may enter into additional joint ventures or partnerships in the future. Joint venture investments and partnerships may involve risks not otherwise present for investments made solely by the Company, including: (i) the Company may not control the joint ventures; (ii) the Company’s joint venture partners may not agree to distributions that it believe are appropriate; (iii) where the Company does not have substantial decision-making authority, it may experience impasses or disputes with the Company’s joint venture partners on certain decisions, which could require it to expend additional resources to resolve such impasses or disputes, including litigation or arbitration; (iv) the Company’s joint venture partners may become insolvent or bankrupt, fail to fund their share of required capital contributions or fail to fulfil their obligations as a joint venture partner; (v) the arrangements governing the Company’s joint ventures may contain certain conditions or milestone events that may never be satisfied or achieved; (vi) the Company’s joint venture partners may have business or economic interests that are inconsistent with the Company’s and may take actions contrary to the Company’s interests; (vii) the Company may suffer losses as a result of actions taken by the Company’s joint venture partners with respect to the Company’s joint venture investments; and (viii) it may be difficult for the Company to exit a joint venture if an impasse arises or if the Company desires to sell its interest for any reason. Any of the foregoing risks could have a material adverse effect on the Company’s business, financial condition and results of operations. In addition, the Company may, in certain circumstances, be liable for the actions of its joint venture partners.
48
The Company may not realize the benefits of its growth strategy, which could have an adverse effect on its business.
As part of its growth strategy, the Company will continue in its existing efforts to go deeper in existing states and initiate new efforts to expand its footprint, and brands. Such expansion is dependent on availability of capital funding, continuing to enter into successful business arrangements and receiving satisfactory regulatory and shareholder approvals, as required. The failure to successfully implement its strategic initiatives could have a material adverse effect on the Company’s business and results of operations.
Risks Related to the Common Shares
The Company’s voting control is concentrated.
Mr. Jason Wild, the Companyʼs Executive Chairman of the Board, beneficially owns, directly or indirectly, or exercises control or direction over shares representing approximately 31.04% of the voting capital stock of the Company as of March 14, 2024. As a result, Mr. Wild exerts significant control over matters that may be put forward for the consideration of all the Company shareholders, including for example, the approval of a potential business combination or consolidation, a liquidation or sale of all or substantially all of the Companyʼs assets, electing members to the Board, and adopting amendments to the Companyʼs constating documents, including its articles of incorporation, as amended (the “Articles”) and by-laws.
The Preferred Shares have a liquidation preference over the Common Shares, which could limit the Company’s ability to make distributions to the holders of Common Shares.
In the event of liquidation, dissolution or winding up of the Company, whether voluntary or involuntary, or upon any other return of capital or distribution of the assets of the Company among its shareholders, in each case for the purposes of winding up its affairs, the Preferred Shares are entitled to receive any distribution available to be paid out of the assets of the Company before the Proportionate Voting Shares, Common Shares and non-participating non-voting exchangeable shares in the capital of the Company ("Exchangeable Shares"). Accordingly, should the Company be liquidated, dissolved or wound-up, the Company may be unable to make any distribution to the holders of the Common Shares.
An investor may face liquidity risks with an investment in the Common Shares.
The Common Shares are listed on the TSX and the OTCQX, however, there can be no assurance that an active and/or liquid market for Common Shares will develop or be maintained and an investor may find it difficult to resell any securities of the Company. For example, given the heightened risk profile associated with cannabis in the United States, capital markets participants may be unwilling to assist with the settlement of trades for U.S. resident securityholders of companies with operations in the U.S. cannabis industry, which may prohibit or significantly impair the ability of securityholders in the United States to trade the Company's securities. In the event residents of the United States are unable to settle trades of the Company's securities, this may affect the pricing of such securities in the secondary market, the transparency and availability of trading prices and the liquidity of these securities.
The price of the Common Shares may be volatile, and may be adversely affected by the price of cannabis.
The market price of the Common Shares may be subject to wide price fluctuations, and the price of the Common Shares, as well as the Companyʼs financial results, may be significantly and adversely affected by a decline in the price of cannabis. There is currently no established market price for cannabis and the price of cannabis is affected by several factors beyond the Companyʼs control. For example, price fluctuations may be in response to many factors, including variations in the operating results of the Company and its subsidiaries, divergence in financial results from analysts’ expectations, changes in earnings estimates by stock market analysts, changes in the business prospects for the Company and its subsidiaries, general economic conditions, legislative changes, community support for the cannabis industry and other events and factors outside of the Companyʼs control. In addition, stock markets have from time-to-time experienced extreme price and volume fluctuations, which, as well as general economic and political conditions, could adversely affect the market price for the Common Shares.
Additional issuances of the Company’s securities may result in dilution.
The Company may issue additional securities in the future, which may dilute a shareholder of the Company's holdings in the Company. The Companyʼs Articles permit the issuance of an unlimited number of Proportionate Voting Shares, Exchangeable Shares, Preferred Shares and Common Shares, and shareholders of the Company will have no pre-emptive rights in connection with such further issuance. The Board have discretion to determine the price and the terms of issue of further issuances, and such terms could include rights, preferences and privileges superior to those existing holders. Moreover, additional Common Shares will be issued by the Company on the exercise of options under the Companyʼs stock option plan (the "Stock Option Plan") and upon the exercise of outstanding warrants and upon the conversion of Proportionate Voting Shares, Exchangeable
49
Shares and Preferred Shares. To the extent holders of the Company’s stock options or other convertible securities convert or exercise their securities and sell the Common Shares they receive, the trading price of the Common Shares may decrease due to the additional amount of Common Shares available in the market. The Company cannot predict the size or nature of future issuances or the effect that future issuances and sales of Common Shares will have on the market price of the Common Shares. Issuances of a substantial number of additional Common Shares, or the perception that such issuances could occur, may adversely affect prevailing market prices for the Common Shares. With any additional issuance of Common Shares, investors will suffer dilution to their voting power and economic interest in the Company.
Sales of substantial amounts of Common Shares may have an adverse effect on the market price of the Common Shares.
Sales of a substantial number of Common Shares in the public market could occur at any time. These sales, or the market perception that the holders of a large number of Common Shares intend to sell Common Shares, could reduce the market price of Common Shares.
The Company’s management will continue to have broad discretion over the use of the proceeds the Company receives in its public offerings, private placements, warrant exercises and loans, as applicable, and might not apply the proceeds in ways that increase the value of your investment.
The Company’s management will continue to have broad discretion to use the net proceeds from its public offerings, private placements warrant exercises and loans, as applicable, and you will be relying on the judgment of the Company’s management regarding the application of these proceeds. The Company’s management might not apply the Company’s net proceeds in ways that ultimately increase the value of your investment. Because of the number and variability of factors that will ultimately influence how the Company uses net proceeds from its public offerings and other financing transactions, the Company’s use of proceeds may vary substantially from their currently intended use. If the Company does not invest or apply the net proceeds from its public offerings, private placements, warrant exercises and loans in ways that enhance shareholder value, it may fail to achieve the expected financial results, which could cause its share price to decline.
Risks related to potential disqualification of equity holders by regulatory authorities.
An individual with an ownership interest in the Company could become disqualified from having such ownership interest in the Company under a U.S. state cannabis agency’s interpretation of the relevant state laws and regulations if such owner is convicted of a certain type of felony or fails to meet the residency requirements, if any, for owning equity in a company like the Company The loss of such equity holder could potentially have a material adverse effect on the Company.
The Company does not intend to pay dividends on its Common Shares for the foreseeable future and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of the Common Shares.
The Company’s policy is to retain earnings to finance the development and enhancement of its products and to otherwise reinvest in the Companyʼs businesses. Therefore, the Company does not anticipate paying cash dividends on Common Shares in the foreseeable future. Any decision to declare and pay dividends in the future will be made at the discretion of the Board and will depend on, among other things, financial results, cash requirements, contractual restrictions and other factors that the Board may deem relevant. As a result, investors may not receive any return on investment in Common Shares unless they sell them for a share price that is greater than that at which such investors purchased them.
“Penny stock” rules may make buying or selling the Company’s securities difficult which may make its securities less liquid and make it harder for investors to buy and sell such securities.
Trading in the Company’s securities is subject to the SEC’s “penny stock” rules and it is anticipated that trading in the Company’s securities will continue to be subject to the penny stock rules for the foreseeable future. The SEC has adopted regulations that generally define a penny stock to be any equity security that has a market price of less than $5.00 per share, subject to certain exceptions. These rules require that any broker-dealer who recommends the Company’s securities to persons other than prior customers and accredited investors must, prior to the sale, make a special written suitability determination for the purchaser and receive the purchaser’s written agreement to execute the transaction. Unless an exception is available, the regulations require the delivery, prior to any transaction involving a penny stock, of a disclosure schedule explaining the penny stock market and the risks associated with trading in the penny stock market. In addition, broker-dealers must disclose commissions payable to both the broker-dealer and the registered representative and current quotations for the securities they offer. The additional burdens imposed upon broker-dealers by these requirements may discourage broker-dealers from recommending transactions in the Company’s securities, which could severely limit the liquidity of such securities and consequently adversely affect the market price for such securities.
50
“Cannabis related business” rules or policies may restrict certain financial institutions from holding the Company’s securities which may make its securities less liquid.
Certain financial institutions have implemented “cannabis related business” rules or policies that prohibit or restrict the trading in cannabis related securities. Trading in the Company securities may be prohibited by certain financial institutions and therefore the Company’s securities may have limited liquidity. Holders of the Company securities may face limitations or be unable to deposit their securities with a brokerage institution.
General Risk Factors
The Company may not be able to obtain necessary permits and authorizations.
The Company may be required to obtain and maintain certain permits, licenses and approvals in the jurisdictions where its products are manufactured and/or sold. There can be no assurance that the Company will be able to obtain or maintain any necessary licenses, permits or approvals. Any material delay or inability to receive these items is likely to delay and/or inhibit the Companyʼs ability to conduct its business, and would have an adverse effect on its business, financial condition and results of operations.
Due to the uncertainty regarding the implementation of the CARES Act and other legislation related to COVID-19, and their application to businesses in the cannabis industry there is a risk that that a determination could be made that the Company is not eligible for the ERC distributions it has received, which may negatively impact the Company.
On March 27, 2020, the Coronavirus Aid, Relief, and Economic Security Act ("CARES Act") was signed into law, aimed at providing emergency assistance and health care for individuals, families, and businesses affected by the COVID-19 pandemic and generally supporting the U.S. economy. The CARES Act, among other things, includes provisions relating to refundable payroll tax credits, deferment of employer social security payments, net operating loss carryback periods, alternative minimum tax credit refunds, and modifications to the net interest deduction limitations. In December 2022, the Company filed for an Employee Retention Tax Credit (“ERC”) distribution in the amount of approximately $14,903. ERC distributions are refundable tax credits for 50% of qualified wages paid to employees during the pandemic. A company is eligible for the ERC if it has not received a Paycheck Protection Program loan under the CARES Act and (i) its operations have been fully or partially suspended because of COVID-19 or (ii) its gross receipts in a calendar quarter in 2020 declined by more than 50% from the same period in 2019.
No formal determination has been made regarding the Company’s eligibility to receive ERC or if companies in the cannabis industry are generally eligible to receive ERC. There is a risk that a determination could be made that the Company, due to its operations or the nature of its business, is not eligible for the ERC distributions it has received. Additionally, there is a risk that if this determination is made that the Company may not be able to repay the funds in the required timeframe and could be subject to additional penalties, which may negatively impact the Company.
The Company may be subject to litigation, which could divert the attention of management and cause the Company to expend significant resources.
The Company may become party to litigation from time to time in the ordinary course of business which could adversely affect its business. Should any litigation in which the Company becomes involved be determined against the Company, such a decision could adversely affect the Companyʼs ability to continue operating and the market price for Common Shares. Even if the Company is involved in litigation and wins, litigation can redirect significant resources.
The Company faces risks and hazards that may not be covered by insurance.
The Companyʼs business is subject to a number of risks and hazards generally, including adverse environmental conditions, accidents, disputes and changes in the regulatory environment. Such occurrences could result in damage to assets, personal injury or death, environmental damage, delays in operations, monetary losses and possible legal liability.
Although the Company maintains insurance to protect against certain risks in such amounts as it considers to be reasonable, its insurance does not cover all the potential risks associated with its operations. The Company may also be unable to maintain insurance to cover these risks at economically feasible premiums. Insurance coverage may not continue to be available or may not be adequate to cover any resulting liability. Moreover, insurance against risks such as environmental pollution or other hazards encountered in the operations of the Company is not generally available on acceptable terms. Losses from these events may cause the Company to incur significant costs that could have a material adverse effect upon its financial performance and results of operations.
51
The Company has incurred and will continue to incur substantial costs as a result of operating as a public company in Canada and the United States, and its management will continue to devote substantial time to new compliance initiatives.
The Common Shares are listed on the TSX under the ticker symbol “TSND.” The Common Shares also trade over the counter in the United States on OTCQX under the ticker symbol “TSNDF.” As a public company, the Company incurs substantial legal, accounting, and other expenses that it would not incur as a private company. For example, the Company is subject to the reporting requirements of the Exchange Act, the applicable requirements of the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act and the rules and regulations of the SEC. The Exchange Act and Securities Act (Ontario) require, among other things, that the Company file annual, quarterly, and current reports with respect to its business, financial condition and results of operations. Compliance with these rules and regulations increase the Company’s legal and financial compliance costs and increase demand on its systems, particularly after the Company is no longer an emerging growth company. In addition, as a public company, the Company may be subject to shareholder activism, which can lead to additional substantial costs, distract management and impact the manner in which the Company operates its business in ways it cannot currently anticipate. As a result of disclosure of information in filings required of a public company, the Company’s business and financial condition are more visible, which may result in threatened or actual litigation, including by competitors.
The Company is currently an “emerging growth company” within the meaning of the Securities Act, and to the extent the Company has taken advantage of certain exemptions from disclosure requirements available to emerging growth companies, this could make its securities less attractive to investors and may make it more difficult to compare the Company’s performance with other public companies.
The Company is an “emerging growth company” within the meaning of the Securities Act, as modified by the JOBS Act, and the Company may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in the Company’s periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. As a result, the Company’s shareholders may not have access to certain information they may deem important. the Company could be an emerging growth company for up to five years, although circumstances could cause the Company to lose that status earlier, including if the market value of its Common Shares held by non-affiliates exceeds $700,000 as of the last business day of the Company’s most recent second fiscal quarter, in which case the Company would no longer be an emerging growth company as of the following fiscal year. The Company cannot predict whether investors will find its securities less attractive because it will rely on these exemptions. If some investors find the Company’s securities less attractive as a result of its reliance on these exemptions, the trading prices of its securities may be lower than they otherwise would be, there may be a less active trading market for the securities and the trading prices of its securities may be more volatile.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such an election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Item 1B. Unresolved Staff Comments
None.
52
Item 1C. Cybersecurity
Risk management and strategy
The Company has implemented and maintain various information security processes designed to identify, assess, and manage material risks from cybersecurity threats to our critical computer networks, third party hosted services, communications systems, hardware and software, and our critical data, including intellectual property, confidential information that is proprietary, strategic or competitive in nature, and patient data (“Information Systems and Data”).
The Company’s cybersecurity function, led by our Chief Information Officer (“CIO”) and Director of IT Operations, helps identify, assess, and manage the Company’s cybersecurity threats and risks. The Company’s CIO has over twenty-five years of technology leadership experience including infrastructure design and management, software development, system implementation, cybersecurity, and network design administration. The Company’s CIO has proven success in data protection for systems and has supported a complex global public enterprise environment with operations in over 200 countries. The Company’s Director of IT Operations has over ten years of experience developing and leading healthcare solutions and has led the development in web and infrastructure design, serverless architecture, database integrations, SMS, mass-email and mass phone notification solutions. The Company’s Director of IT Operations has dealt directly with numerous cyber security threats, breaches, detection and containment activities and strategies under strict requirements and expectations for HIPAA covered entities. The Company’s cybersecurity team works in partnership with our managed security service provider (“MSSP”) and managed service provider (“MSP”) to help identify, assess, and respond to risks from cybersecurity threats. The Company’s Security Incident Response Team leverages a cybersecurity managed detection and response partner, which monitors certain of our endpoints, cloud infrastructure, and networks for irregular activity.
Depending on the environment and system, the Company implements and maintains various technical, physical, and organizational measures, processes, standards, and policies designed to manage and mitigate material risks from cybersecurity threats to our Information Systems and Data, including, for example: an incident response plan, monitoring and response services in certain environments and systems, system access controls for certain systems, an employee cybersecurity training program, asset management and recovery processes, penetration testing, dark web monitoring, monthly environment risk reviews, external threat intelligence reports, regular digital infrastructure scans, internally reported threats, and cybersecurity insurance.
The Company’s assessment and management of material risks from cybersecurity threats are integrated into the Company’s overall risk management processes. For example, cybersecurity risk is identified in the Company’s risk register detailing certain known and potential risk items; the Company’s IT operations department works with our MSSP/MSP vendor partners to review certain identified threats and our cybersecurity practices in an effort to prioritize and mitigate cybersecurity threats that are more likely to lead to a material impact on our business; and our CIO regularly presents to senior management, the Board and Audit Committee to evaluate material risks relative to our overall business objectives.
The Company uses third-party service providers to assist us from time to time to identify, assess, and manage material risks from cybersecurity threats including, for example, managed cybersecurity service providers, cybersecurity software providers, penetration testing firms, and professional services firms, including legal counsel.
The Company uses third-party service providers to perform a variety of functions throughout our business, such as enterprise resource planning, point of sale, eCommerce, wholesale sales, and web hosting. We have a vendor management program to manage cybersecurity risks associated with our use of these providers. Our vendor management program includes completion of a cybersecurity questionnaire for certain vendors designed to help us evaluate the potential cybersecurity risks associated with our use of the vendor. Depending on the nature of the services provided, the sensitivity of the Information Systems and Data at issue, and the identity of the provider, our vendor management process may involve different levels of assessment designed to help identify cybersecurity risks associated with a provider and impose contractual obligations related to cybersecurity on the provider.
For a description of the risks from cybersecurity threats that may materially affect the Company and how they may do so, see the risk factors under Item 1A. "Risk Factors" in this Annual Report, including “The Company (and the third parties upon which
53
it relies) faces physical security risks, as well as risks related to its information technology systems, potential cyber-attacks, and security breaches.”.
Governance
The Board addresses the Company’s cybersecurity risk management as part of its general oversight function. The Company’s Audit Committee is responsible for overseeing the Company’s cybersecurity risk management processes, including oversight and mitigation of risks from cybersecurity threats.
The Company's cybersecurity risk assessment and management processes are implemented and maintained by certain Company management, the CIO and the Director of IT Operations. The CIO is responsible for hiring appropriate personnel, helping to integrate cybersecurity risk considerations into the Company’s overall risk management strategy, and communicating key priorities to relevant personnel. The CIO is responsible for approving budgets, helping prepare for cybersecurity incidents, approving cybersecurity processes, and reviewing security assessments and other security-related reports.
Our cybersecurity incident response and vulnerability management processes are designed to escalate certain cybersecurity incidents to members of management depending on the circumstances, including the security management department, CIO, Chief Financial Officer (“CFO”), Chief Executive Officer (“CEO”) and others. The security management department, CIO, CFO, CEO, and others work with the Company’s incident response team to help the Company mitigate and remediate cybersecurity incidents of which they are notified. In addition, the Company’s incident response and vulnerability management processes includes reporting to the Board for certain cybersecurity incidents.
The Board receives regular reports from the CIO concerning the Company’s significant cybersecurity threats and risks and the processes the Company has implemented to address them. These reports also include materials related to certain cybersecurity trends we have identified, potential cybersecurity threats and risk, and proposed mitigation measures.
Item 2. Properties
TerrAscend's corporate headquarters is located in King of Prussia, Pennsylvania and the Issuer’s registered office is in Mississauga, Ontario, Canada. In addition, the Company has various other offices, manufacturing, cultivation and/or processing facilities and dispensaries across the United States. The Company believes that its current offices and facilities are suitable and adequate to meet its current needs. The Company intends to add new facilities or expand existing facilities as it adds employees, and it believes that suitable additional or substitute space will be available as needed to accommodate any such expansion of the Company's operations.
The following tables set forth the Company’s material physical properties as of March 14, 2024.
Corporate Properties |
||||
Type |
|
Location |
|
Leased / Owned |
|
|
|
|
|
Office |
|
IHC Management LLC / TerrAscend Corp. King of Prussia, PA |
|
Leased* |
|
|
|
|
|
Office |
|
Corporate Office (West) Santa Rosa, CA |
|
Leased |
|
|
|
|
|
Office |
|
Corporate Office (Midwest) Troy, MI |
|
Leased |
|
|
|
|
|
Production and Storage Properties |
||||
Type |
|
Location |
|
Leased / Owned |
|
|
|
|
|
Manufacturing, Cultivation |
|
Ilera – Grow PA Waterfall, PA |
|
Owned* |
|
|
|
|
|
Manufacturing, Cultivation |
|
TerrAscend NJ LLC Boonton, NJ |
|
Owned |
54
|
|
|
|
|
Cultivation |
|
State Flower San Francisco, CA |
|
Leased |
|
|
|
|
|
Manufacturing, Cultivation |
|
HMS Health Hagerstown, Maryland |
|
Owned |
|
|
|
|
|
Cultivation |
|
Gage Cannabis Co. Warren, MI |
|
Owned |
|
|
|
|
|
Cultivation, Processing |
|
Gage Cannabis Co. Harrison, MI |
|
Owned |
|
|
|
|
|
Cultivation, Processing |
|
Gage Cannabis Co. Monitor, MI
|
|
Owned |
55
Retail Properties |
||||
Type |
|
Location |
|
Leased / Owned |
|
|
|
|
|
Dispensary |
|
Cookies Toronto, Canada |
|
Leased |
|
|
|
|
|
Dispensary |
|
The Apothecarium – Plymouth Plymouth Meeting, PA |
|
Leased* |
|
|
|
|
|
Dispensary |
|
The Apothecarium – Lancaster Lancaster, PA |
|
Leased* |
|
|
|
|
|
Dispensary |
|
The Apothecarium – Thorndale Thorndale, PA |
|
Leased* |
|
|
|
|
|
Dispensary |
|
KCR Dispensary – Allentown Allentown, PA |
|
Owned* |
|
|
|
|
|
Dispensary |
|
KCR Dispensary – Bethlehem Bethlehem, PA |
|
Leased* |
|
|
|
|
|
Dispensary |
|
KCR Dispensary – Stroudsburg Stroudsburg, PA |
|
Leased* |
|
|
|
|
|
Dispensary |
|
The Apothecarium – Phillipsburg Phillipsburg, NJ |
|
Owned |
|
|
|
|
|
Dispensary |
|
The Apothecarium – Maplewood Maplewood, NJ |
|
Leased |
|
|
|
|
|
Dispensary |
|
The Apothecarium – Lodi Lodi, NJ |
|
Leased |
|
|
|
|
|
Dispensary |
|
AMMD Cumberland, MD |
|
Leased* |
|
|
|
|
|
Dispensary |
|
Peninsula Salisbury, MD |
|
Leased |
|
|
|
|
|
Dispensary |
|
Blue Ridge Parkville, MD |
|
Leased |
|
|
|
|
|
Dispensary |
|
Blue Ridge Nottingham, MD |
|
Leased |
|
|
|
|
|
Dispensary |
|
Herbiculture Burtonsville, MD |
|
Leased |
|
|
|
|
|
Dispensary |
|
The Apothecarium – Castro San Francisco, CA |
|
Leased |
|
|
|
|
|
Dispensary |
|
The Apothecarium – Marina San Francisco, CA |
|
Leased |
|
|
|
|
|
Dispensary |
|
The Apothecarium – Soma San Francisco, CA |
|
Leased |
|
|
|
|
|
Dispensary |
|
The Apothecarium – Berkeley |
|
Leased |
56
|
|
Berkeley, CA |
|
|
|
|
|
|
|
Dispensary |
|
The Apothecarium – Capitola Capitola, CA |
|
Leased |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Cookies Ann Arbor, MI |
|
Leased* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Adrian, MI |
|
Leased* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Battle Creek, MI |
|
Leased* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Burton, MI |
|
Leased* |
Retail Properties |
||||
Type |
|
Location |
|
Leased / Owned |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Cookies Detroit, MI |
|
Owned* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Ferndale, MI |
|
Leased* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Grand Rapids, MI |
|
Leased* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Cookies Kalamazoo, MI |
|
Leased* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Kalamazoo, MI |
|
Owned* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Lansing, MI |
|
Leased* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Traverse City, MI |
|
Leased* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Addison, MI |
|
Owned* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Buchanan, MI |
|
Owned* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Camden, MI |
|
Owned* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Edmore, MI |
|
Leased* |
|
|
|
|
|
Dispensary |
|
Pinnacle Emporium Morenci, MI |
|
Owned* |
57
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Bay City, MI |
|
Owned* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Oxford, MI |
|
Leased* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Lemonnade Centerline, MI |
|
Owned* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Coleman, MI |
|
Owned* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Sturgis, MI |
|
Owned* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Mt. Morris, MI |
|
Owned* |
Retail Properties |
||||
Type |
|
Location |
|
Leased / Owned |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Cookies Jackson, MI |
|
Leased* |
|
|
|
|
|
Dispensary |
|
Gage Cannabis Co. Detroit, MI |
|
Owned* |
* Property or lease on the property is subject to an encumbrance as described below.
Not yet operational.
Properties Subject to an Encumbrance
Ilera Term Loan
The following have been pledged as collateral to secure obligations of TerrAscend’s subsidiary, WDB Holding PA, under a senior secured term loan, entered into on December 18, 2020, by and between WDB Holding PA and a syndicate of lenders, originally in the principal amount of $120,000, bearing an interest rate of 12.875% per annum and maturing on December 17, 2024 ("Ilera Term Loan"): (i) Ilera – Grow PA, Waterfall, Pennsylvania and (ii) KCR Dispensary – Allentown, Allentown, Pennsylvania. The interests of WDB Holding PA in the leases for the following properties have been pledged as collateral to secure the obligations WDB Holding PA under the same loan: (i) IHC Management LLC / TerrAscend Corp. Office., King of Prussia, Pennsylvania, (ii) The Apothecarium Dispensary – Plymouth, Plymouth Meeting, Pennsylvania, (iii) The Apothecarium Dispensary – Lancaster, Lancaster, Pennsylvania, (iv) The Apothecarium Dispensary – Thorndale, Pennsylvania, (v) KCR Dispensary – Bethlehem, Pennsylvania, (vi) KCR Dispensary – Stroudsburg, Pennsylvania. The loan is secured by the assets of the Companyʼs Pennsylvania business. As a result of Ilera entering into a commercial loan with Stearns Bank N.A. on June 26, 2023 that included a senior encumbrance on the Ilera – Grow PA, Waterfall, Pennsylvania property, the pledged security interest in the Ilera - Grow PA, Waterfall under the senior secured term loan herein was subordinated to a junior position.
Stearns Loan
The following have been pledged as collateral to secure obligations of TerrAscend’s subsidiary, Ilera, under a commercial loan entered into on June 26, 2023, by and between Ilera and Stearns Bank., in the principal amount of $25,000, bearing an interest rate of prime plus 2.25% and maturing on December 26, 2024 ("Stearns Loan"):(i) a senior encumbrance on the Ilera – Grow PA, Waterfall, Pennsylvania property, (ii) a junior encumbrance on the license for Ilera -Grow PA, Waterfall, Pennsylvania, and (iii) a senior lien on the AMMD dispensary in Cumberland, Maryland.
58
Chicago Atlantic Term Loan
The following have been pledged as collateral to secure obligations of the Company under a credit agreement originally entered into on November 22, 2021, by and between TerrAscend Growth Corp. and Chicago Atlantic, LLC, originally in the principal amount of $55,000, bearing an interest rate of the greater of the prime rate plus 6.00%, and 13.00% per annum and maturing on November 1, 2024 ("Chicago Atlantic Term Loan") : all TerrAscend MI properties, leases, and licenses.
Pelorus Term Loan
The following have been pledged as collateral to secure obligations of the Company under a single-draw senior secured term loan with an aggregate principal amount of $45,478, entered into on October 11, 2022 ("Pelorus Term Loan"), by and between TerrAscend NJ, LLC, HMS Health, HMS Processing, HMS Hagerstown, LLC ("HMS Hagerstown") and Pelorus Fund REIT, LLC ("Pelorus"): (i) TerrAscend NJ assets, unless otherwise excluded by the loan agreement; (ii) HMS Health; (iii) HMS Processing; and (iv) and HMS Hagerstown, (v) Cultivation and Processing – Boonton, NJ, (vi) The Apothecarium Dispensary – Phillipsburg, NJ, (vii) Cultivation and Processing – Hagerstown, MD, and (viii) interests of TerrAscend NJ, LLC in the leases of The Apothecarium – Maplewood, NJ and The Apothecarium – Lodi, NJ. The Pelorus Term Loan bears interest at a variable rate tied to the one month Secured Overnight Financing Rate ("SOFR"), subject to a base rate, plus 9.5% maturing on October 11, 2027.
See the section titled "Item 7, Management's Discussion and Analysis of Financial Condition and Results of Operations—Debt Facilities" of this Annual Report for more information regarding the Company's indebtedness.
Item 3. Legal Proceedings
Legal Proceedings
In the ordinary course of business, the Company is involved in a number of lawsuits incidental to its business, including litigation related to intellectual property, product liability, employment, and commercial matters. Although it is difficult to predict the ultimate outcome of these matters, management believes that any ultimate liability would not have a material adverse effect on the Company’s Consolidated Balance Sheets or results of operations. Other than as set out below, at December 31, 2023, there were no pending lawsuits that could reasonably be expected to have a material effect on the results of the Company’s consolidated financial statements, except for the proceedings described below.
Pure X Litigation
On August 9, 2023, AEY Capital LLC (“AEY”), a licensed subsidiary of the Company, filed a lawsuit in Oakland County Circuit Court (the “Oakland Court”) against Pure X, LLC (“Pure X”) seeking damages in the amount of $14,969 (the “AEY Claim”). The AEY Claim alleges breach of contract, quantum meruit/unjust enrichment, account stated and statutory conversion. AEY’s alleged damages are related to Pure X’s failure to pay for various cannabis products sold by AEY. This matter is still pending.
Item 4. Mine Safety Disclosures
Not applicable.
59
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
The Common Shares were traded on the CSE, under the ticker symbol “TER,” from May 3, 2017 until July 4, 2023, when TerrAscend commenced trading of its Common Shares on the TSX under the ticker symbol “TSND”. On October 22, 2018, TerrAscend’s Common Shares began trading on OTCQX under the ticker symbol “TRSSF,” which was changed to “TSNDF,” effective July 6, 2023. Any over-the-counter market quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
Shareholders
The Company had 443 shareholders of record as of March 14, 2024. This does not include shares held in the name of a broker, bank or other nominees (typically referred to as being held in “street name”).
Dividends
The Company has not declared any dividends or made any distributions. Furthermore, the Company has no current intention to declare dividends on its Common Shares in the foreseeable future. Any decision to pay dividends on its Common Shares in the future will be at the discretion of the Board of Directors and will depend on, among other things, the Company’s results of operations, current and anticipated cash requirements and surplus, financial condition, any future contractual restrictions and financing agreement covenants, solvency tests imposed by corporate law and other factors that the Board of Directors may deem relevant.
Stock Performance Graph
The following shall not be deemed incorporated by reference into any of the Company's other filings under the Exchange Act, except to the extent the Company specifically incorporates it by reference into such filings.
The following compares he cumulative total return for an investment of $100 in TerrAscend's Common Shares, Russell 2000 Index and a selected peer group of companies for the five years ended December 31, 2023. The comparison assumes all
60
dividends have been reinvested (if any). Effective July 4, 2023, the Company commenced trading its Common Shares on the TSX, under the ticker symbol "TSND".
The comparisons in this graph below are based upon historical data and are not indicative of, nor intended to forecast, future performance of the Common Shares.
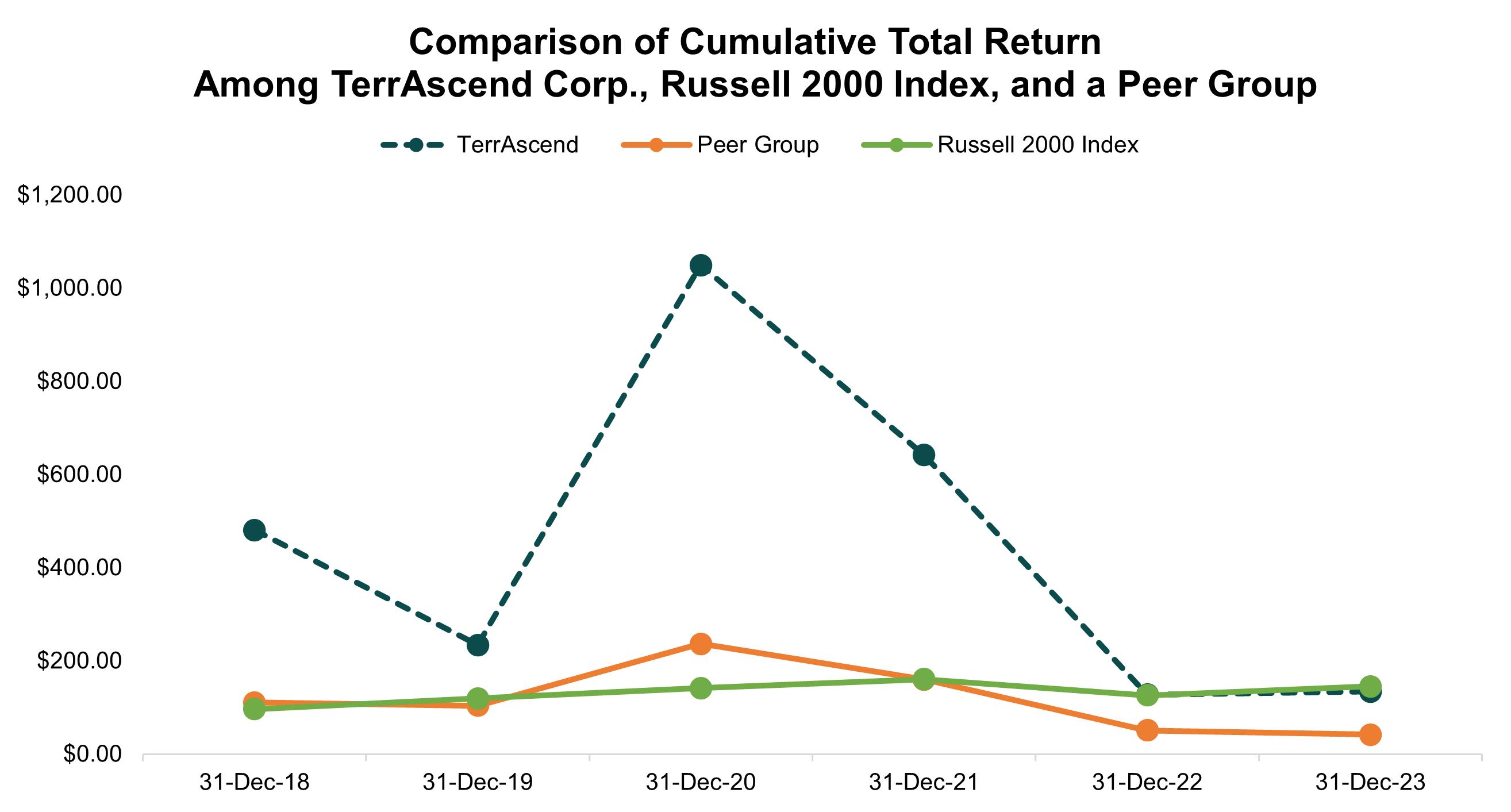
The following specific companies were included in the peer group:
Recent Sales of Unregistered Securities
The following information represents securities sold by the Company during the fiscal year ended December 31, 2023, which were not registered under the Securities Act. Included are new issues, securities issued in exchange for property, services or other securities, securities issued upon conversion from other Company share classes and new securities resulting from the modification of outstanding securities. The Company sold all of the securities listed below pursuant to the exemption from registration provided by Section 4(a)(2) of the Securities Act, or Regulation D or Regulation S promulgated thereunder.
Common Shares
During fiscal year ended December 31, 2023, 1,468,521 Common Shares were issued to holders of the 2023 RSUs (as defined below) upon settlement at fair value prices on the dates of issuance between $1.40 and $1.72 per share.
61
In 2023, 532,185 Common Shares were issued to an individual shareholder at price range between $1.28 and $1.60 per share as a result of a liability settlement.
Other Issuances
During the year ended December 31, 2023, 2,191,627 options to purchase Common Shares were granted to certain Company employees as additional compensation pursuant to TerrAscend's Stock Option Plan at various exercise prices between $1.13 and $2.06 per share. These options vest annually over a period of four years.
During the year ended December 31, 2023, 2,387,275 RSUs (“2023 RSU”) were granted to various employees and members of the board of directors as compensation pursuant to TerrAscend’s RSU Plan at grant prices between $1.40 and $1.72 per share. Each 2023 RSU entitles the holder to receive one Common Share. Of the RSUs issued, 602,183 vested immediately on the grant date, and 866,338 vested on December 31, 2023. The remainder vest annually over three to four years.
Use of Proceeds from Initial Public Offering of Common Shares
Not applicable.
Purchase of Equity Securities by the Issuer and Affiliated Purchasers
None.
Item 6. [Reserved]
62
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of the Company's financial condition and results of operations should be read in conjunction with the Company's audited consolidated financial statements as of December 31, 2023 and December 31, 2022 and for the years ended December 31, 2023, December 31, 2022, and December 31, 2021 ("Consolidated Financial Statements") appearing elsewhere in this Annual Report. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report, including information with respect to the Company's plans and strategy for its business, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth under "Risk Factors" appearing elsewhere in this Annual Report its actual results could differ materially from the results described in or implied by the forward looking information contained in this Annual Report and in the following discussion and analysis.
Unless otherwise noted, dollar amounts in this Item 2 are in thousands of U.S. dollars.
Overview
The Company is a leading North American cannabis company. The Company has vertically integrated licensed operations in Pennsylvania, New Jersey, Michigan, Maryland and California. In addition, the Company has retail operations in Ontario, Canada with a majority-owned dispensary in Toronto, Ontario, Canada. Notwithstanding the fact that various states in the U.S. have implemented medical marijuana laws or have otherwise legalized the use of cannabis, the use of cannabis remains illegal under U.S. federal law for any purpose, by way of the Controlled Substances Act.
The Company operates under one operating segment, which is the cultivation, production and sale of cannabis products.
The Company owns a portfolio of operating businesses, including:
Recent Developments
63
Subsequent Transactions
64
Components of Results of Operations
The following discussion sets forth certain components of our Consolidated Statements of Comprehensive Income (Loss) as well as factors that impact those items.
Revenue, net
The Company generates revenue from the sale of cannabis products, brands, and services to the United States and Canadian markets. Revenues consist of wholesale and retail sales in the legal medical and adult-use market across Canada and in several U.S. states where cannabis has been legalized for medical or adult-use cannabis.
Cost of sales
Cost of sales primarily consists of expenses related to providing cannabis products and services to the Company's customers, including personnel-related expenses, the depreciation of property and equipment, amortization of acquired intangible assets, certain royalties, and other overhead costs.
Operating Expenses
General and administrative
General and administrative ("G&A") expenses consist primarily of personnel costs related to finance, human resources, legal, certain royalties, and other administrative functions. Additionally, G&A expenses include professional fees to third parties, as well as marketing expenses. Moreover, G&A expenses includes share-based compensation on options, restricted stock units and warrants. The Company expects that G&A expenses will increase in absolute dollars as the business grows.
Amortization and depreciation
Amortization and depreciation includes the amortization of intangible assets. Amortization is calculated on a straight-line basis over the following terms:
Brand intangibles- indefinite lives |
Indefinite useful lives |
Brand intangibles- definite lives |
3 years |
Software |
5 years |
Licenses |
5-30 years |
Non-compete agreements |
3 years |
Depreciation of property and equipment is calculated on a straight-line basis over the estimated useful life of the asset using the following terms:
Buildings and improvements |
15-30 years |
Land |
Not depreciated |
Machinery & equipment |
5-15 years |
Office furniture & production equipment |
3-5 years |
Right of use assets |
Lease term |
Assets in process |
Not depreciated |
Impairment of intangible assets and goodwill
Goodwill and indefinite lived intangible assets are reviewed for impairment annually and whenever there are events or changes in circumstances that indicate that the carrying amount has been impaired. The Company first performs a qualitative assessment. If based on the results of a qualitative assessment it has been determined that it is more likely than not that the fair value of a reporting unit exceeds its carrying value, an additional quantitative impairment test is performed which compares the carrying value of the reporting unit to its estimated fair value. If the carrying value exceeds the estimated fair value, an impairment is recorded.
Definite lived intangible assets are tested for impairment when there are indications that an asset may be impaired. When indicators of impairment exist, the Company performs a quantitative impairment test which compares the carrying value of the
65
assets for intangible assets to their estimated fair values. If the carrying value exceeds the estimated fair value, an impairment is recorded.
Impairment of property and equipment
The Company evaluates the recoverability of property and equipment whenever events or changes in circumstances indicate that the carrying value of the asset, or asset group, may not be recoverable. When the Company determines that the carrying value of the long-lived asset may not be recoverable based upon the existence of one or more indicators, the assets are assessed for impairment based on the estimate of future undiscounted cash flows expected to result from the use of the asset and its eventual disposition. If the carrying value of an asset exceeds its estimated future undiscounted cash flows, an impairment loss is recorded for the excess of the asset’s carrying value over its fair value.
(Gain) loss from revaluation of contingent consideration
As a result of some of its acquisitions, the Company recognizes a contingent consideration payable, which is an obligation to transfer additional assets to the seller if future events occur. The liability is revalued at the end of each reporting period to determine its fair value. A gain or loss is recognized as a result of the revaluation.
(Gain) loss on fair value of warrants and purchase option derivative asset
The Company issues warrant liability that are remeasured to fair value at the end of each reporting unit using the Black-Scholes Option Pricing Model ("Black-Scholes Model"). A gain or loss is recognized as a result of the revaluation.
Finance and other expenses
Finance and other expenses consist primarily of interest expense on the Company's outstanding debt obligations.
Transaction and restructuring costs
Transaction costs include costs incurred in connection with the Company's acquisitions, such as expenses related to professional fees, consulting, legal and accounting. Restructuring costs are those costs associated with severance and restructuring of business units.
Unrealized and realized foreign exchange loss
Unrealized and realized foreign exchange loss represents the loss recognized on the remeasurement of USD denominated cash and other assets recorded in the Canadian dollars functional currency at the Company's Canadian operations.
Unrealized and realized gain on investments
The Company accounts for its investment in equity securities without readily determinable fair values using a valuation technique which maximizes the use of relevant observable inputs, with subsequent holding changes in fair value recognized in unrealized gain or loss on investments in the Consolidated Statement of Comprehensive Loss.
Provision for income taxes
Provision for income taxes consists of U.S. federal and state income taxes in certain jurisdictions in which the Company conducts business.
Results from Operations - Years ended December 31, 2023, December 31, 2022, and December 31, 2021
The following tables represent the Company’s results from operations for the years ended December 31, 2023, December 31, 2022, and December 31, 2021:
Revenue, net
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Revenue, net |
|
$ |
317,328 |
|
|
$ |
247,829 |
|
|
$ |
194,210 |
|
$ change |
|
$ |
69,499 |
|
|
$ |
53,619 |
|
|
|
|
|
% change |
|
|
28 |
% |
|
|
28 |
% |
|
|
|
|
66
Revenue increased from $247,829 to $317,328 during the year ended December 31, 2023 as compared to the year ended December 31, 2022 driven by an increase of $55,938 in retail revenue and $13,561 in wholesale revenue. The increase was primarily a result of growth from adult-use sales in New Jersey and implementation of adult-use sales in Maryland during 2023, along with the acquisitions of AMMD, Peninsula, Blue Ridge, and Herbiculture in Maryland.
The increase in net revenue from $194,210 to $247,829 for the year ended December 31, 2022 as compared to December 31, 2021 was due to an increase of $96,900 in retail revenue. The increase in retail revenue was mainly due to adult-use sales in New Jersey, which commenced in April of 2022, as well as the Gage and Pinnacle Acquisitions in Michigan. Retail dispensaries increased from thirteen at December 31, 2021 to thirty-one at December 31, 2022. The retail revenue increase was partially offset by a decrease of $43,281 in wholesale revenue from $107,091 at December 31, 2021 to $63,810 at December 31, 2022, which was mainly related to challenging market dynamics in Pennsylvania.
Cost of Sales
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Cost of sales |
|
$ |
156,645 |
|
|
$ |
137,243 |
|
|
$ |
79,465 |
|
Non-cash adjustment of inventory |
|
|
985 |
|
|
|
9,082 |
|
|
|
2,243 |
|
Total cost of sales |
|
$ |
157,630 |
|
|
$ |
146,325 |
|
|
$ |
81,708 |
|
$ change |
|
$ |
11,305 |
|
|
$ |
64,617 |
|
|
|
|
|
% change |
|
|
8 |
% |
|
|
79 |
% |
|
|
|
|
Cost of sales as a % of revenue |
|
|
50 |
% |
|
|
59 |
% |
|
|
42 |
% |
The increase in cost of sales for the year ended December 31, 2023 as compared to the year ended December 31, 2022 is mainly due to increased sales as noted above. The reduction in cost of sales as a percentage of revenue is driven by increased yields in New Jersey, improved utilization in Maryland, lower costs in Pennsylvania as a result of scaling back the cultivation facility, and reduced discounting combined with improved verticalization in Michigan.
The increase in cost of sales for the year ended December 31, 2022 as compared to December 31, 2021 was driven mainly by the Gage Acquisition, as well as an increase in New Jersey due to the increase in adult-use sales which commenced during 2022. The increase in cost of sales as a percentage of revenue was due to lower volumes in Pennsylvania leading to under-absorption, primarily related to lower wholesale flower sales, operational challenges at the Company's cultivation facility in Frederick, Maryland as the Company transitioned to its Hagerstown location, and higher costs of sales as a percentage of revenue as a result of the acquisition of Gage Growth in Michigan.
In addition, during the year ended December 31, 2022, the Company wrote down its inventory by $9,082 mainly due to write downs of inventory to lower of cost or market which was related to the Company's operational reconfiguration of its cultivation facility in Pennsylvania and write downs of inventory related to the vape recall in Pennsylvania. During the year ended December 31, 2021, the Company recorded impairment of $2,243 mainly related to inventory at the Company's Pennsylvania operations that did not meet quality standards.
67
General and Administrative Expense (G&A)
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
General and administrative expense |
|
$ |
115,189 |
|
|
$ |
115,588 |
|
|
$ |
75,107 |
|
$ change |
|
$ |
(399 |
) |
|
$ |
40,481 |
|
|
|
|
|
% change |
|
|
0 |
% |
|
|
54 |
% |
|
|
|
|
The decrease in G&A expenses for the year ended December 31, 2023 as compared to the year ended December 31, 2022 was primarily driven by a bad debt expense of $10,331 taken in 2022 offset by an increase in G&A expenses in 2023 primarily due to acquisitions in Maryland and the implementation of adult-use sales in the state.
The increase in G&A expenses for the year ended December 31, 2022 as compared to the year ended December 31, 2021 was primarily due to increased office and general expense of $15,909, increased salary and wages of $14,558, and increased sales and marketing expense of $8,460, which was mainly a result of the Gage and Pinnacle Acquisitions in Michigan.
Amortization and Depreciation Expense
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Amortization and depreciation |
|
$ |
9,433 |
|
|
$ |
9,658 |
|
|
$ |
5,533 |
|
$ change |
|
$ |
(225 |
) |
|
$ |
4,125 |
|
|
|
|
|
% change |
|
|
-2 |
% |
|
|
75 |
% |
|
|
|
|
There were no significant changes in amortization and depreciation expense for the year ended December 31, 2023 as compared to the year ended December 31, 2022.
The increase in amortization and depreciation expense for the year ended December 31, 2022 as compared to December 31, 2021 was primarily due to the Gage Acquisition and Pinnacle Acquisitions. In connection with such acquisitions, the Company acquired cultivation and processing, as well as retail licenses which are amortized over a 15-year period.
Impairment of intangible assets
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Impairment of intangible assets |
|
$ |
51,303 |
|
|
$ |
140,727 |
|
|
$ |
3,633 |
|
$ change |
|
$ |
(89,424 |
) |
|
$ |
137,094 |
|
|
|
|
|
% change |
|
|
-64 |
% |
|
|
3774 |
% |
|
|
|
|
During the year ended December 31, 2023, the Company performed an impairment analysis over its definite lived retail licenses in California and determined that its carrying value was greater than its fair value, and therefore, recorded impairment of $15,518. In the Michigan market, during each of the years ended December 31, 2023 and 2022, the Company performed an impairment analysis over its indefinite lived intangible assets acquired through the Gage Acquisition as the changes in the market expectations of cash flows as well as increased competition and supply in the state were determined to be indicators of impairment. As a result of the impairment analysis performed in 2023, the Company determined that it was more likely than not that the carrying value of the Gage brand name and Gage banner was greater than its fair value, and therefore, recorded impairment of $34,185 and $ 1,600 for Gage brand name and Gage banner, respectively.
As a result of the impairment analysis performed in 2022, the Company determined that it was more likely than not that the carrying value of its definite lived retail and cultivation and processing licenses was greater than its fair value, and therefore recorded impairment of $79,462 and $42,065 for the retail license and the cultivation and processing licenses, respectively, reducing both the carrying values to $nil at December 31, 2022. Additionally, the Company recorded impairment of its indefinite lived brand intangible assets acquired through the Gage Acquisition of $19,200.
The impairment recorded during the year ended December 31, 2021 relates to the write-off of intellectual property at the Company’s Arise business.
68
Impairment of goodwill
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Impairment of goodwill |
|
$ |
4,690 |
|
|
$ |
170,357 |
|
|
$ |
5,007 |
|
$ change |
|
$ |
(165,667 |
) |
|
$ |
165,350 |
|
|
|
|
|
% change |
|
|
-97 |
% |
|
|
3302 |
% |
|
|
|
|
During the year ended December 31, 2023, it was determined that it was more likely than not that the California reporting unit's fair value was less than its carrying value. As a result of this quantitative test, the Company recorded impairment of goodwill of $4,690 related to the State Flower Acquisition, reducing the carrying value of goodwill within the California reporting unit, to $nil.
During the year ended December 31, 2022, as it was determined that it was more likely than not that the Michigan reporting unit's fair value was less than its carrying value, a one-step goodwill quantitative impairment test was performed. As a result of the quantitative test, TerrAscend recorded impairment of goodwill of $170,357 related to the Gage Acquisition and Pinnacle Acquisition, reducing the carrying value of goodwill within the Michigan reporting unit, to $nil.
The impairment recorded during the year ended December 31, 2021 relates to TerrAscend’s Florida reporting unit as the Company determined that the estimated cash flows of the Arise business did not support the carrying value of the intangible assets and goodwill. As a result, the Company recorded impairment to reduce the balance of goodwill at its Florida reporting unit to $nil.
(Gain) loss from revaluation of contingent consideration
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
(Gain) loss from revaluation of contingent consideration |
|
$ |
(645 |
) |
|
$ |
(1,061 |
) |
|
$ |
3,584 |
|
$ change |
|
$ |
416 |
|
|
$ |
(4,645 |
) |
|
|
|
|
% change |
|
|
-39 |
% |
|
|
-130 |
% |
|
|
|
|
For the year ended December 31, 2023, TerrAscend recognized a gain of $645 due to a reduction in the contingent liability for the Peninsula Acquisition, which was a result of the increase in TerrAscend's share price from the grant date.
TerrAscend recognized a gain on revaluation of contingent consideration of $1,061 for the year ended December 31, 2022 as compared to a loss of $3,584 during the year ended December 31, 2021. During the year ended December 31, 2022, the fair value of the contingent consideration related to the KCR Acquisition was reduced to $nil, as it was determined that it was more likely than not that the earnout criteria would not be met.
Gain on extinguishment of debt
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Gain on extinguishment of debt |
|
$ |
- |
|
|
$ |
(4,153 |
) |
|
$ |
- |
|
$ change |
|
$ |
4,153 |
|
|
$ |
(4,153 |
) |
|
|
|
|
% change |
|
|
-100 |
% |
|
|
-100 |
% |
|
|
|
|
There was no extinguishment of debt for the year ended December 31, 2023.
Pursuant to the Canopy Debt Settlement Arrangement, on December 9, 2022, TerrAscend and certain of its subsidiaries extinguished certain debt obligations with the Canopy USA Entities, including all the principal and interest on the amounts outstanding thereunder, in exchange for 24,601,467 Exchangeable Shares of TerrAscend at a notional price of $3.74 per Exchangeable Share. Additionally, in accordance with Accounting Standards Codification ("ASC") 815 Derivatives and Hedging, the 22,474,130 original warrants to acquire Common Shares of TerrAscend were modified at a weighted average exercise price of $4.45 per Common Share. The Exchangeable Shares and Modified Canopy Warrants were considered in the calculation for extinguishment of the debt obligations, including all principal and interest on the amounts outstanding thereafter (refer to Note 9 included in Item 8, "Financial Statements and Supplementary Data" in this Annual Report on Form 10-K for
69
further details). As a result of these transactions, TerrAscend recorded a gain on extinguishment of debt of $4,153 for the year ended December 31, 2022.
Gain on fair value of warrants and purchase option derivative asset
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Gain on fair value of warrants and purchase option derivative assets |
|
$ |
(322 |
) |
|
$ |
(58,523 |
) |
|
$ |
(57,904 |
) |
$ change |
|
$ |
58,201 |
|
|
$ |
(619 |
) |
|
|
|
|
% change |
|
|
-99 |
% |
|
|
1 |
% |
|
|
|
|
The warrant liability was remeasured to fair value at December 31, 2023 using the Black-Scholes Model. TerrAscend recognized a gain of $372 during the year ended December 31, 2023 as a result of the declining share price from the warrant issuance date, as compared to December 31, 2023. During the year ended December 31, 2023, the purchase option derivative asset expired and was recorded as a loss of $50.
The warrant liability was previously remeasured to fair value at December 31, 2022 using the Black-Scholes Model. The Company recognized a gain during the twelve months ended December 31, 2022 as a result of the reduction of the Company's share price from December 31, 2021 as compared to December 31, 2022, as well as from warrants exercised during the twelve months ended December 31, 2022. The combined impact resulted in a gain on fair value of warrants of $59,341 for the twelve months ended December 31, 2022.
During the year ended December 31, 2022, the purchase option derivative asset was remeasured to fair value using the Monte Carlo simulation model resulting in a loss of $818.
The warrant liability was previously remeasured to fair value at December 31, 2021 using the Black-Scholes Model. TerrAscend recognized a gain during the twelve months ended December 31, 2021 as a result of the reduction of the Common Share price from December 31, 2020 as compared to December 31, 2021, as well as from warrants exercised during the twelve months ended December 31, 2021. The combined impact resulted in a gain on fair value of warrants of $58,158 for the twelve months ended December 31, 2021.
For the year ended December 31, 2021, the purchase option derivative asset was remeasured to fair value using the Monte Carlo simulation model resulting in a loss of $254.
Finance and other expenses
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Finance and other expenses |
|
$ |
37,041 |
|
|
$ |
35,893 |
|
|
$ |
27,849 |
|
$ change |
|
$ |
1,148 |
|
|
$ |
8,044 |
|
|
|
|
|
% change |
|
|
3 |
% |
|
|
29 |
% |
|
|
|
|
The increase in finance and other expenses for the year ended December 31, 2023 as compared to the year ended December 31, 2022 was primarily due to the increase of accretion expense related to the change to the effective interest rate method for the Company's debt facilities.
The increase in finance and other expenses for the year ended December 31, 2022 as compared to the year ended December 31, 2021 was primarily due to an increase of $14,070 in interest expense recognized primarily related to the loans acquired as part of the Gage Acquisition, as well as fees to modify its term loan acquired through the Gage Acquisition and amendment fees related to the Ilera Term Loan, both which did not meet the criteria to be capitalized, totaling $2,507. This increase in finance and other expenses was offset by other income of $9,440 related to an ERC received as a result of the CARES Act which provides for a refundable tax credit for businesses that continued to pay employees while shut down due to the COVID-19 pandemic or had significant declines in gross receipts from March 13, 2020 to December 31, 2021.
70
Transaction and restructuring costs
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Transaction and restructuring costs |
|
$ |
344 |
|
|
$ |
1,445 |
|
|
$ |
3,111 |
|
$ change |
|
$ |
(1,101 |
) |
|
$ |
(1,666 |
) |
|
|
|
|
% change |
|
|
-76 |
% |
|
|
-54 |
% |
|
|
|
|
The decrease of transaction and restructuring costs for the year ended December 31, 2023 was primarily related to fees incurred on the Gage and Pinnacle Acquisitions during the year ended December 31, 2022.
Similarly, the decrease of transaction and restructuring costs for the year ended December 31, 2022 as compared to December 31, 2021 was related to higher legal costs on the KCR and HMS acquisitions for the year ended December 31, 2021.
(Gain) loss on lease termination
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
(Gain) loss on lease termination |
|
$ |
(1,217 |
) |
|
$ |
- |
|
|
$ |
3,278 |
|
$ change |
|
$ |
(1,217 |
) |
|
$ |
(3,278 |
) |
|
|
|
|
% change |
|
|
-100 |
% |
|
|
-100 |
% |
|
|
|
|
During the year ended December 31, 2023, the Company entered into a lease termination agreement in Florida. As a result of the lease termination, the Company recorded a gain on lease termination of $1,217.
During the year ended December 31, 2021, the Company entered into a lease termination agreement with the landlord at its 22,000 square foot facility in Frederick, Maryland to enable the Company to terminate the lease prior to the end of the lease term. As a result of the lease termination, the Company recorded a loss on lease termination of $3,278.
Unrealized and realized foreign exchange (gain) loss
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Unrealized and realized foreign exchange (gain) loss |
|
$ |
(53 |
) |
|
$ |
712 |
|
|
$ |
4,654 |
|
$ change |
|
$ |
(765 |
) |
|
$ |
(3,942 |
) |
|
|
|
|
% change |
|
|
-107 |
% |
|
|
-85 |
% |
|
|
|
|
The change in unrealized foreign exchange (gain) loss from a loss of $712 for the year ended December 31, 2022 to a gain of $53 for the year ended December 31, 2023 was a result of TerrAscend changing its functional currency to U.S. dollars from Canadian dollars in July 2023.
The decrease in unrealized foreign exchange loss for the year ended December 31, 2022 as compared to December 31, 2021 was a result of the remeasurement of U.S. dollar denominated liabilities recorded in Canadian dollar functional currency at the Company's Canadian operations.
71
Unrealized and realized loss (gain) on investments
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Unrealized and realized loss (gain) on investments |
|
$ |
2,603 |
|
|
$ |
(43 |
) |
|
$ |
(6,192 |
) |
$ change |
|
$ |
2,646 |
|
|
$ |
6,149 |
|
|
|
|
|
% change |
|
|
-6153 |
% |
|
|
-99 |
% |
|
|
|
|
During the year ended December 31, 2023, TerrAscend purchased a controlling interest in Cookies Canada. Under ASC 805, Business Combinations, the previously held equity interest is remeasured at fair value at the date TerrAscend acquired the controlling interest, which resulted in recording a loss of $1,149. Additional loss of $ 814 was related to a license in Georgia.
The gain on investment during the year ended December 31, 2022 was related to the revaluation of the investments acquired through the Gage Acquisition.
The increase in the unrealized gain in the year ended December 31, 2021 relates to the acquisition of the remaining 90% of investment in KCR during the first half of 2021.
Provision for (benefit from) income taxes
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Provision for (benefit from) income taxes |
|
$ |
23,453 |
|
|
$ |
(10,783 |
) |
|
$ |
28,877 |
|
$ change |
|
$ |
34,236 |
|
|
$ |
(39,660 |
) |
|
|
|
|
% change |
|
|
317 |
% |
|
|
-137 |
% |
|
|
|
|
The increase in tax expense for the year ended December 31, 2023 as compared to December 31, 2022 is primarily related to decreases in the pre-tax book loss due to operational results compared to December 31, 2022, as well as the goodwill impairment that reduced tax expense for the Company in 2022. The Company's effective tax rate was (40.0)% for the year ended December 31, 2023, as compared to 3.5% for the year ended December 31, 2022. The Company's effective tax rate for the year ended December 31, 2023 was higher than the federal statutory tax rate of 21% primarily driven by the impact of Section 280E of the Code on the Company’s taxes due.
The decrease in tax expense for the year ended December 31, 2022 as compared to December 31, 2021 is primarily related to the pre-tax book loss due to the impairment of goodwill taken during the year ended December 31, 2022. The Company's effective tax rate was 3.5% for the year ended December 31, 2022, as compared to 64.8% for the year ended December 31, 2021. The Company's effective tax rate for the year ended December 31, 2022 was lower than the federal statutory tax rate of 21% primarily driven by the impairment of goodwill and the increase in the valuation allowance.
The Company's effective tax rate can vary each reporting period depending on, among other factors, the geographic and business mix of the Company's earnings, changes to the valuation allowance, and permanently non-deductible expenses. Certain of these and other factors, including the Company's history and projections of pre-tax earnings, are considered in assessing the Company's ability to realize any deferred tax assets including net operating losses.
Liquidity and Capital Resources
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
|
|
$ |
|
|
$ |
|
||
Cash and cash equivalents |
|
|
22,241 |
|
|
|
26,158 |
|
Restricted Cash |
|
|
3,106 |
|
|
|
605 |
|
Current assets |
|
|
102,889 |
|
|
|
121,993 |
|
Non-current assets |
|
|
563,629 |
|
|
|
579,594 |
|
Current liabilities |
|
|
207,000 |
|
|
|
137,905 |
|
Non-current liabilities |
|
|
218,778 |
|
|
|
242,511 |
|
Working capital |
|
|
(104,111 |
) |
|
|
(15,912 |
) |
Total shareholders' equity |
|
|
240,740 |
|
|
|
321,171 |
|
72
The calculation of working capital provides additional information and is not defined under GAAP. The Company defines working capital as current assets less current liabilities. This measure should not be considered in isolation or as a substitute for any standardized measure under GAAP.
Liquidity and going concern
At December 31, 2023, the Company had an accumulated deficit of $704,162 and cash and cash equivalents of $22,241. During the year ended December 31, 2023, the Company incurred a net loss from continuing operations of $82,286. Additionally, as of December 31, 2023, the Company had a net capital deficiency. Therefore, the Company expects that it may need additional capital to continue to fund its operations.
The aforementioned indicators raise substantial doubt about the Company's ability to continue as a going concern for at least one year from the issuance of these Consolidated Financial Statements included in this Annual Report on Form 10-K. The Company believes this concern is mitigated by steps it has taken, or intends to take to improve its operations and cash position, including: (i) identifying access to future capital required to meet the Company’s on-going obligations, (ii) improved cashflow growth from the Company's consolidated operations, particularly TerrAscend's operations in New Jersey and most recently Maryland with conversion to adult-use sales, and (iii) various cost and efficiency improvements. The financial statements do not include any adjustments relating to the recoverability and classification of asset carrying amounts or the amounts of and classification of liabilities that may result should the Company be unable to continue as a going concern.
Since its inception, the Company's primary sources of capital have been through the issuance of equity securities or debt facilities, and the Company has received aggregate net proceeds from such transactions totaling $653,006 as of December 31, 2023.
The Company expects to fund any additional future requirements through the following sources of capital:
Capital requirements
The Company has $206,453 in principal amounts of loans payable at December 31, 2023. Of this amount, $141,946 are due in the next twelve months.
The Company has entered into leases for certain premises and offices for which it owes monthly lease payments. The Company has $94,618 in lease obligations. Of this amount, $9,506 are due in the next twelve months.
The Company's undiscounted contingent consideration payable is $6,446 at December 31, 2023, all of which is due in the next twelve months. The contingent consideration payable relates to the Apothecarium 2019 Acquisition, State Flower Acquisition, and Peninsula Acquisition. Contingent consideration is based upon the potential earnout of the underlying business unit and is measured at fair value using a projection model for the business and the formulaic structure for determining the consideration under the agreement. The contingent consideration is revalued at the end of each reporting period. The contingent considerations of State Flower and Apothecarium 2019 Acquisitions were paid to the sellers on January 19, 2024.
During the year ended December 31, 2020, the Company expensed $7,500 related to amounts payable to an entity controlled by the minority shareholders of TerrAscend NJ, LLC pursuant to services surrounding the granting of certain licenses. The final payment of $3,750 was made in July, 2023.
At December 31, 2023, the Company had accounts payable and accrued liabilities of $49,897 and corporate income taxes payable of $4,775.
73
The Company does not have any off-balance sheet arrangements that have, or are reasonably likely to have, a current or future effect on the Company's results of operations or financial condition, including and without limitation, such consideration as liquidity and capital resources.
The Company intends to meet its capital commitments through any or all of the sources of capital noted above. The Company's objective with respect to its capital management is to ensure it has sufficient cash resources to maintain its ongoing operations and finance future obligations.
Debt facilities
Ilera Term Loan
On December 18, 2020, WDB Holding PA, a subsidiary of TerrAscend, entered into the Ilera Term Loan, a senior secured term loan with a syndicate of lenders in the amount of $120,000. The Ilera Term Loan is solely secured by Ilera. The Ilera Term Loan bears interest at 12.875% per annum and matures on December 17, 2024. Subject to certain conditions of the agreement, the Company has the ability to increase the facility by up to $30,000. WDB Holding PA's obligations under the Ilera Term Loan and related transaction documents are guaranteed by the Company, TerrAscend USA, Inc. ("TerrAscend USA"), and certain subsidiaries of WDB Holding PA, and secured by TerrAscend USA's equity interest in WDB Holding PA and substantially all of the assets of WDB Holding PA and the subsidiary guarantors party thereto. The loan can be refinanced at the option of the WDB Holding PA after 18 months from the closing date subject to a premium payment due. Of the total proceeds received, $105,767 was used to satisfy the remaining Ilera earn-out payments.
On April 28, 2022, the Ilera Term Loan was amended to provide WDB Holding PA with greater flexibility by resetting the minimum consolidated interest coverage ratio levels that must be satisfied at the end of each measurement period and extending the date in which WDB Holding PA is required to deliver its budget for the fiscal year ending December 31, 2021. In addition, the no-call period was extended from 18 months to 30 months, subject to a premium payment. This modification was not considered extinguishments of debt under ASC 470, Debt.
On November 11, 2022, WDB Holding PA, the Company, TerrAscend USA and the subsidiary guarantors party to the Ilera Term Loan and the PA Agent (on behalf of the required lenders) entered into an amendment to the Ilera Term Loan, pursuant to which the parties agreed that WDB Holding PA’s obligation to maintain the consolidated interest coverage ratio as set forth in the Ilera Term Loan for the period ended September 30, 2022, shall not apply, subject to certain conditions, including (but not limited to) an obligation to enter into a subsequent amendment agreement on or before December 15, 2022, documenting certain enhancements and amendments to the Ilera Term Loan. In addition, WDB Holding PA offered a prepayment of $5,000 pro rata to all lenders holding outstanding loans thereunder at a price equal to 103.22% of the principal amount prepaid, plus accrued and unpaid interest.
On December 21, 2022, WDB Holding PA completed an amendment to reduce the Company’s principal debt by $35,000 and annual interest expense by $5,000. The Company agreed to make a $35,000 payment at the original prepayment price of 103.22% to par, and agreed to use commercially reasonable efforts to add certain collateral to the Ilera Term Loan, collectively by March 15, 2023. The amendment further provided that should WDB Holding PA fail to maintain the prescribed interest coverage ratio, the Company shall be required to deposit funds, as outlined in the amendment, into a restricted account, and no event of default shall occur. This amendment was not considered an extinguishment of debt under ASC 470 Debt.
On March 15, 2023, WDB Holding PA, in exchange for a fee in the amount of 1% of the then outstanding principal loan balance, agreed to an amendment to, among other things: (i) extend the obligation date to prepay the Company’s debt from March 15, 2023 to June 30, 2023, during which WDB Holding PA agreed to use commercially reasonable efforts to add additional collateral to the Ilera Term Loan, (ii) increase the amount of debt to be reduced by up to $37,000, subject to certain reductions in amount based on meeting certain time based milestones, at a prepayment price of 103.22% to par, and (iii) extend the next test date in respect of the interest coverage ratio until June 30, 2023. This amendment was not considered an extinguishment of debt under ASC 470, Debt.
On April 14, 2023, WDB Holding PA entered into an amendment to the Ilera Term Loan to, among other things, (i) permit changes necessary for the TSX Transaction (as defined in the Ilera Term Loan), and (ii) to waive certain tax provisions.
On June 8, 2023, June 15, 2023, and June 29, 2023, WBD Holding PA made repayments of principal in the amounts of $7,896, $442, and $28,236, respectively.
On June 22, 2023, WDB Holding PA entered into a further amendment to the Ilera Term Loan to, among other things, (i) extend the next test date for the interest coverage ratio from June 30, 2023 to September 30, 2023, and (ii) amend the terms for
74
which WDB Holding PA may incur certain indebtedness and liens. This amendment was not considered extinguishment of debt under ASC 470, Debt.
On October 2, 2023, the Company made a mandatory prepayment of the Ilera Term Loan of $1,500 at the original prepayment price of 103.22% to par.
On December 4, 2023, the parties entered into an amendment that requires WDB Holding PA to make a prepayment of $4,800 by January 2, 2024 and a prepayment of $3,200 by April 30, 2024, at the prepayment price of 100% to par.
As of December 31, 2023, there was an outstanding principal amount of $76,927 under the Ilera Term Loan. Subsequently, the Company paid an additional $4,800 which resulted in a current outstanding principal amount of $72,127.
Chicago Atlantic Term Loan
In connection with the Gage Acquisition, the Company assumed the senior secured term loan Chicago Atlantic Term Loan, with an acquisition date fair value of $53,857. The credit agreement bears interest at a rate equal to the greater of (i) the Prime Rate plus 7% or (ii) 10.25%. The term loan was payable monthly and had a maturity date of November 30, 2022. The Chicago Atlantic Term Loan was secured by a first lien on all Gage Growth assets. As a result of the amendment, the Company paid a loan amendment fee of $1,109 which was capitalized.
On August 10, 2022, the Chicago Atlantic Term Loan was amended as a result of the corporate restructure in conjunction with the Gage Acquisition. The amendment to the Chicago Atlantic Term Loan includes the addition of a borrower and guarantor under the term loan and a right of first offer in favor of the administrative agent for a refinancing of the term loan. This amendment was not considered extinguishment of debt under ASC 470, Debt.
On November 29, 2022, the Company repaid $30,000 outstanding principal amount on the Chicago Atlantic Term Loan. On November 30, 2022, the remaining loan principal amount of $25,000 on the Chicago Atlantic Term Loan was amended (the "Amended Chicago Atlantic Term Loan"). The Amended Chicago Atlantic Term Loan bears interest on $25,000 at a per annum rate equal to the greater of (i) the U.S. "prime rate" plus 6.00%, and (ii) 13.0% and matures on November 1, 2024. Commencing on May 31, 2023, the Company will make monthly principal repayments of 0.40% of the aggregate principal amount outstanding. Additionally, the unpaid principal amount of the loan shall bear paid in kind interest at a rate of 1.50% per annum. No prepayment fees are owed if the Company voluntarily prepays the loan after 18 months. If such prepayment occurs prior to 18 months, a prepayment fee equal to all of the interest on the loans that would be due after the date of such prepayment, is owed. Under the Amended Chicago Atlantic Term Loan, the Company has the ability to borrow incremental term loans of $30,000 at the option of the Company and subject to consents from the required lenders. The additional $30,000 incremental term loans available under the amendment have not been drawn as of December 31, 2023. This loan represents a loan syndication, and therefore the Company assessed each of the lenders separately under ASC 470, Debt to determine if this represents a modification, or an extinguishment of debt. For three of the four remaining lenders, it was determined that this was a modification. For the remaining lender, it was determined that this represented an extinguishment of debt and therefore the fees paid to the lenders on modification were expensed. As a result of this transaction, the Company expensed $1,907 of fees paid to the lenders and third parties as they did not meet the criteria for capitalization under ASC 470, Debt. As of December 31, 2023, there was an outstanding principal amount of $24,611.
On June 9, 2023, the Company agreed to an amendment among other things, to (i) permit changes necessary for the TSX Transaction (as defined therein) and (ii) to permit certain indebtedness and waive certain tax provisions. This amendment was not considered extinguishment of debt under ASC 470, Debt.
Pinnacle Loans
The Pinnacle Acquisition purchase price included two promissory notes in an aggregate amount of $10,000 to pay down all Pinnacle liabilities and encumbrances. The promissory note matures on June 30, 2023 and bears interest rates of 6%. On June 27, 2023, Spartan Partners Properties, LLC, agreed to an amendment among other things, to extend the obligation date of the
75
loan until December 1, 2023. As of December 31, 2023, there was an outstanding principal amount of $5,582 on the two promissory notes. The obligations are subject to restructuring upon the resolution of indemnity claims.
Pelorus Term Loan
On October 11, 2022, subsidiaries of TerrAscend, among others, entered into a loan agreement with Pelorus for the single-draw senior secured Pelorus Term Loan in an aggregate principal amount of $45,478. The Pelorus Term Loan bears interest at a variable rate tied to the one month SOFR, subject to a base rate, plus 9.5%, with interest-only payments for the first 36 months. The base rate is defined as, on any day, the greatest of (a) 2.5%, (b) the effective federal funds rate in effect on such day plus 0.5%, and (c) SOFR in effect on such day. The obligations of the borrowers under the Pelorus Term Loan are guaranteed by the Company, TerrAscend USA and certain other subsidiaries of TerrAscend and are secured by all of the assets of the Company's Maryland and New Jersey businesses, including certain real estate in Maryland and New Jersey, but excludes all Maryland dispensaries. The Pelorus Term Loan matures on October 11, 2027.
On April 17, 2023, TerrAscend NJ, LLC agreed to an amendment to the Pelorus Term Loan to, among other things, (i) permit changes necessary for the TSX Transaction (as defined therein), and (ii) to waive certain tax provisions.
On June 22, 2023, TerrAscend NJ, LLC further agreed to an amendment to the Pelorus Term Loan to permit the Company to incur certain indebtedness. This amendment was not considered an extinguishment of debt under ASC 470, Debt. As of December 31, 2023, there was an outstanding principal amount of $45,478 under the Pelorus Term Loan.
Stearns Loan
On June 26, 2023, the Company closed on the Stearns Loan, a $25,000 commercial loan with Stearns Bank, secured by the Company's cultivation facility in Pennsylvania and its AMMD dispensary in Cumberland, Maryland. The Company was required to hold $5,000 on deposit in a restricted account, of which $2,500 of the restricted cash was released on July 28, 2023 upon meeting certain criteria pursuant to the terms of the Stearns Loan. The Stearns Loan bears interest at a rate of prime plus 2.25% and matures on December 26, 2024. The proceeds from the loan were used to pay down the Company's higher interest rate debt, thereby lowering the Company's overall interest expense. As of December 31, 2023, there was an outstanding principal amount of $24,809 under the Stearns Loan.
Maryland Acquisition Loans.
On June 28, 2023, in connection with the Peninsula Acquisition, the Company assumed existing indebtedness in the form of a promissory note in the amount of $7,698, which matures on June 28, 2025. The promissory note bears interest at a rate of 8.25%. The Company will make monthly payments of principal and interest totaling $157 beginning on July 28, 2023. The Company is required to make a mandatory prepayment of 50% of the outstanding principal balance on January 28, 2025. The consideration also included a promissory note in the amount of $3,927. The promissory note interest at a rate of 7.25% and is payable in twelve quarterly installments, maturing on June 28, 2026.
On June 30, 2023, in connection with the Blue Ridge Acquisition, the Company entered into a promissory note in the amount of $3,750 payable in four quarterly installments of accrued interest commencing on September 30, 2023 and twelve equal quarterly installments of principal and accrued interest commencing on September 30, 2024. The remaining amount of the principal and accrued interest is due on June 30, 2027, the maturity date. The promissory note bears interest at a rate of 7.0%.
On July 10, 2023, in connection with the Herbiculture Acquisition, the Company entered into a promissory note in the amount of $5,250. The promissory note bears interest at a rate of 10.50%. Commencing on September 30, 2023, and thereafter until December 31, 2024, all accrued interest during each quarter will be added to the outstanding principal balance on the last day of each fiscal quarter. Beginning on March 31, 2025, and thereafter until March 31, 2026, only interest payments will be due on the last day of each fiscal quarter. The entire outstanding balance of the principal and accrued interest is due on June 30, 2026, the maturity date of the promissory note.
As of December 31, 2023, there was an outstanding principal amount of $19,873 under the promissory notes related to the Maryland acquisitions.
76
Stadium Ventures
In connection with the Gage Acquisition, the Company assumed existing indebtedness in the form of a promissory note in the amount of $4,065, which matures on December 31, 2024. The promissory note bears interest at a rate of 6%. As of December 31, 2023, there was an outstanding principal amount of $1,698 on the promissory note.
Class A Shares of TerrAscend
In connection with the Reorganization (see Note 3), TerrAscend Growth Corp. ("TerrAscend") issued $1,000 of Class A shares with a 20% guaranteed annual dividend ("Class A Shares") to an investor (the “Investor”) pursuant to the terms of a subscription agreement between TerrAscend and the Investor dated April 20, 2023 (the “Subscription Agreement”). Pursuant to the terms of the Subscription Agreement, TerrAscend holds a call right to repurchase all of the Class A Shares issued to the Investor for an amount equal to the sum of: (a) the Repurchase/Put Price (as defined in the Subscription Agreement); plus (b) the amount equal to 40% of the subscription amount less the aggregate dividends paid to the Investor as of the date of the exercise of the option. In addition, the Investor holds a put right that is exercisable at any time after four months’ advanced written notice following the five-year anniversary of the closing of the investment to put all (and only all) of the Class A Shares owned by the Investor to TerrAscend at the Repurchase/Put Price, payable in cash or shares. The instrument is considered as a debt for accounting purposes due to the economic characteristics and risks. As of December 31, 2023, there was an outstanding principal amount of $1,000.
IHC Real Estate LP Loan
On June 26, 2023, the Company acquired the minority interest in IHC Real Estate LP and in connection therewith, the Company entered into a promissory note in the amount of $7,500. The promissory note bore interest at a rate of 15% and matured on January 15, 2024. On June 28, 2023 and July 31, 2023, the Company made a payment of $1,500 and $1,000, respectively. As of December 31, 2023, there was an outstanding principal amount of $5,000 under the promissory note.
On January 15, 2024, the Company paid off all amounts owed under the promissory note with a payment of $5,000.
Bay City Promissory Note
On October 30, 2023, the Company entered into a promissory note in the amount of $1,430. The promissory note bears interest at a rate of 13%. Commencing on November 30, 2023 until July 30, 2024, the Company is required to make a monthly payment of principal and interest. As of December 31, 2023 there was an outstanding principal amount of $1,124 under the promissory note.
Cash Flows
Cash flows provided by (used in) operating activities
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Net cash provided by (used in) operating activities |
|
$ |
27,471 |
|
|
$ |
(26,123 |
) |
|
$ |
(31,815 |
) |
The increase of $53,594 in net cash provided by operating activities for the year ended December 31, 2023 as compared to December 31, 2022 is primarily due to an increase in income from operations and reduced tax payments, partially offset by an increase in working capital.
The decrease in cash used in operating activities for the year ended December 31, 2022 as compared to December 31, 2021 is primarily due to a decrease in income taxes paid from $37,060 during the year ended December 31, 2021 to $9,917 for the year ended December 31, 2022, as well as a decrease in contingent consideration payments made related to the Company's acquisitions in excess of the fair value of the liability recognized at the date of acquisition from $11,394 during the year ended December 31, 2021 to $410 during the year ended December 31, 2022. Excluding these amounts, the Company had net cash used in operating activities of $15,795 for the year ended December 31, 2022 as compared to net cash provided by operating activities of $16,639 for the year ended December 31, 2021. Additionally, the increase in net cash used in operating activities for the year ended December 31, 2022 as compared to December 31, 2021 is due to an increase in loss from operations, excluding non-cash impairment on intangible assets and goodwill, of $57,828 from a profit of $24,831 in the prior year period to a loss of $32,997 in the current year. The decrease in cash used in operating activities is offset by an increase in interest
77
payments made on loans from $21,171 for the year ended December 31, 2021 to $26,840 for the year ended December 31, 2022, primarily due to the loans acquired through the Gage Acquisition and the Pinnacle Acquisition.
The decrease in cash used in operating activities for the year ended December 31, 2021 as compared to December 31, 2020, is primarily due to payments made in excess of the amount of fair value of the contingent consideration payable at the date of the Ilera acquisition on September 16, 2019 of $11,394 for the year ended December 31, 2021 as compared to payments of $56,527 during the year ended December 31, 2020. Excluding these amounts, the Company had net cash used in operating activities of $20,421 and cash provided by operating activities of $19,556 for the years ended December 31, 2021 and December 31, 2020, respectively. The increase in cash used in operating activities during the year ended December 31, 2021 is mainly the result of income tax payments of $37,060 during the year ended December 31, 2021, as compared to $11,204 during the year ended December 31, 2020, and interest payments of $21,171 during the year ended December 31, 2021, as compared to $1,955 during the year ended December 31, 2020. The increase in interest payments is primarily due to the Ilera Term Loan in which the Company received proceeds during December 2020. Excluding these payments, the Company saw an increase in cash provided by operating activities during the year ended December 31, 2021, which is primarily due to increased sales.
Cash flows used in investing activities
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Net cash used in investing activities |
|
$ |
(16,219 |
) |
|
$ |
(27,579 |
) |
|
$ |
(132,421 |
) |
The net cash used in investing activities for the year ended December 31, 2023 primarily relates to $16,789 of cash paid for the acquisition of four dispensaries in Maryland. Additionally, the Company increased the investment in property and equipment by $7,762 during 2023. The Company also paid the success fee of $3,750 related to Alternative Treatment Center licenses issued by the New Jersey Department of Health. These investments were partially offset by proceeds from the sale of the Company's Canadian facility of $14,285.
The net cash used in investing activities for the year ended December 31, 2022 is mainly due to investment in property and equipment of $39,631, primarily related to the buildout of a cultivation site in Maryland, continuing renovations at the Company's Pennsylvania and Michigan cultivation sites, and the completion of the Company's Lodi alternative treatment center in New Jersey. Additionally, the Company had investments in intangible assets of $2,261, primarily related to adult-use licenses in New Jersey, as well as the acquisition of a retail license in Michigan. The cash used in investing activities was offset by cash inflows of $24,716 related to cash and restricted cash acquired through the Gage Acquisition, offset by net cash paid for consideration for the Pinnacle Acquisition of $8,489.
The net cash used in investing activities for the year ended December 31, 2021 primarily relates to cash consideration paid for the acquisitions of KCR and HMS totaling $42,736 and $50,000 related to the purchase of the additional 12.5% of the issued and outstanding equity of TerrAscend NJ, LLC from BWH NJ, LLC and Blue Marble Ventures, LLC. Additionally, the Company had investments in property and equipment of $37,858 primarily related to the buildout of the New Jersey operations and expansions in Pennsylvania cultivation and $1,977 related to deposits paid for the expansion of the cultivation premises in Pennsylvania.
Cash flows (used in) provided by from financing activities
|
|
For the Years Ended |
|
|||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Net cash (used in) provided by financing activities |
|
$ |
(12,500 |
) |
|
$ |
3,719 |
|
|
$ |
182,201 |
|
Net cash used in financing activities for the year ended December 31, 2023 was primarily due to loan principal payment of $50,154 and distributions to minority partners of $11,621 and offset by the cash inflow of transfer with recourse of ERC of $12,677, net proceeds from the commercial loan with Stearns Bank N.A. of $23,869, and net proceeds from private placements of $20,822. Additionally, the Company recorded cash outflow of $5,539 from discontinued operation.
Net cash provided by financing activities for the year ended December 31, 2022, was primarily due to loan net proceeds from Pelorus in the amount of $43,419 and proceeds from exercises of Common Share warrants and stock options of $24,342. The cash provided by financing activities was offset by principal payments on loans and loan modification fees of $42,221 and
78
$4,977, respectively, payments made to non-controlling interests of $7,550, and payments of contingent consideration related to the acquisition of State Flower of $6,630.
Net cash provided by financing activities for the twelve months ended December 31, 2021, was mainly a result of the private placement on January 28, 2021, in which the Company issued 18,115,656 Common Shares at a price of $9.64 (CAD 12.35) per Common Share for total proceeds of $173,477, net of share issuance costs of $1,643. Additionally, during the twelve months ended December 31, 2021, 8,755,339 Common Share warrants were exercised for total proceeds of $21,735 and 1,376,496 stock options were exercised at $0.67-$6.93 (CAD 0.85-$8.52) per unit for total gross proceeds of $5,462. In addition, 1,968 Preferred Share warrants were exercised at $3,000 per unit for total gross proceeds of $3,588. The cash provided by financing activities was offset by payments of contingent consideration related to the acquisition of Ilera of $18,274 and by payments of loan principal of $4,500 related to the KCR loan.
Reconciliation of Non-GAAP Measures
In addition to reporting the financial results in accordance with GAAP, the Company reports certain financial results that differ from what is reported under GAAP. Non-GAAP measures used by management do not have any standardized meaning prescribed by GAAP and may not be comparable to similar measures presented by other companies. The Company believes that certain investors and analysts use these measures to measure a company’s ability to meet other payment obligations or as a common measurement to value companies in the cannabis industry, and the Company calculates (i) Free cash flow from net cash provided by (used in) operating activities from continuing operations less capital expenditures for property and equipment which management believes is an important measurement of the Company's ability to generate additional cash from its business operations, and (ii) Adjusted EBITDA from continuing operations as net (loss) income, adjusted to exclude provision for income taxes, finance expenses, depreciation and amortization, relief of fair value upon acquisition, share-based compensation, gain on extinguishment of debt, restructuring related charges, impairment of good will and intangible assets and certain other items, which management believes is not reflective of the ongoing operations and performance. Such information is intended to provide additional information and should not be considered in isolation or as a substitute for measures of performance prepared in accordance with GAAP.
The Company believes Adjusted EBITDA from continuing operations is a useful performance measure to assess the performance of the Company as it provides more meaningful ongoing operating results by excluding the effects of expenses that are not reflective of the Company’s underlying business performance and other one-time or non-recurring expenses. The table below reconciles net (loss) income to EBITDA from continuing operations and Adjusted EBITDA from continuing operations for the years ended December 31, 2023, December 31, 2022, and December 31, 2021:
79
|
|
|
For the Years Ended |
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
Notes |
|
December 31, 2023 |
|
|
|
December 31, 2022 |
|
|
|
December 31, 2021 |
|
|||
Net (loss) income |
|
|
$ |
(86,730 |
) |
|
|
$ |
(325,351 |
) |
|
|
$ |
6,135 |
|
Loss from discontinued operations |
|
|
|
4,444 |
|
|
|
|
25,949 |
|
|
|
|
9,518 |
|
(Loss) income from continuing operations |
|
|
|
(82,286 |
) |
|
|
|
(299,402 |
) |
|
|
|
15,653 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Add (deduct) the impact of: |
|
|
|
|
|
|
|
|
|
|
|
|
|||
Provision for income taxes |
|
|
|
23,453 |
|
|
|
|
(10,783 |
) |
|
|
|
28,877 |
|
Finance expenses |
|
|
|
35,106 |
|
|
|
|
39,059 |
|
|
|
|
24,121 |
|
Amortization and depreciation |
|
|
|
20,382 |
|
|
|
|
22,624 |
|
|
|
|
12,789 |
|
EBITDA from continuing operations |
(a) |
|
|
(3,345 |
) |
|
|
|
(248,502 |
) |
|
|
|
81,440 |
|
Add (deduct) the impact of: |
|
|
|
|
|
|
|
|
|
|
|
|
|||
Relief of fair value upon acquisition |
(b) |
|
|
— |
|
|
|
|
2,770 |
|
|
|
|
3,465 |
|
Non-cash write downs of inventory |
(c) |
|
|
— |
|
|
|
|
5,894 |
|
|
|
|
449 |
|
Vape recall |
(d) |
|
|
— |
|
|
|
|
2,965 |
|
|
|
|
— |
|
Share-based compensation |
(e) |
|
|
7,707 |
|
|
|
|
12,162 |
|
|
|
|
14,941 |
|
Impairment of goodwill and intangible assets |
(f) |
|
|
55,993 |
|
|
|
|
311,084 |
|
|
|
|
8,640 |
|
(Gain) loss from revaluation of contingent consideration |
(g) |
|
|
(645 |
) |
|
|
|
(1,061 |
) |
|
|
|
3,584 |
|
Restructuring costs and executive severance |
(h) |
|
|
921 |
|
|
|
|
472 |
|
|
|
|
816 |
|
Legal settlements |
(i) |
|
|
746 |
|
|
|
|
623 |
|
|
|
|
1,590 |
|
Other one-time items |
(j) |
|
|
3,808 |
|
|
|
|
5,207 |
|
|
|
|
6,070 |
|
Loan modification fees |
(k) |
|
|
— |
|
|
|
|
2,507 |
|
|
|
|
— |
|
Bad debt expense write offs in Michigan |
(l) |
|
|
— |
|
|
|
|
9,941 |
|
|
|
|
— |
|
Employee Retention Credits and Transfer Fee |
(m) |
|
|
2,236 |
|
|
|
|
(9,440 |
) |
|
|
|
— |
|
Gain on extinguishment of debt |
(n) |
|
|
— |
|
|
|
|
(4,153 |
) |
|
|
|
— |
|
(Gain) loss on lease termination and derecognition of ROU asset |
(o) |
|
|
(1,012 |
) |
|
|
|
1,162 |
|
|
|
|
3,278 |
|
Gain on fair value of warrants and purchase option derivative asset |
(p) |
|
|
(322 |
) |
|
|
|
(58,523 |
) |
|
|
|
(57,904 |
) |
Indemnification asset release |
(q) |
|
|
— |
|
|
|
|
3,973 |
|
|
|
|
4,504 |
|
Impairment of property and equipment |
(r) |
|
|
2,079 |
|
|
|
|
1,089 |
|
|
|
|
312 |
|
(Gain) loss on disposal of fixed assets |
(s) |
|
|
(1,914 |
) |
|
|
|
— |
|
|
|
|
— |
|
Unrealized and realized loss (gain) on investments |
(t) |
|
|
2,603 |
|
|
|
|
(43 |
) |
|
|
|
(6,192 |
) |
Unrealized and realized foreign exchange (gain) loss |
(u) |
|
|
(53 |
) |
|
|
|
712 |
|
|
|
|
4,654 |
|
Adjusted EBITDA from continuing operations |
|
|
$ |
68,802 |
|
|
|
$ |
38,839 |
|
|
|
$ |
69,647 |
|
The table below reconciles net cash provided by (used in) operating activities from continuing operations to free cash flow for the years ended December 31, 2023, December 31, 2022 and December 31, 2021:
|
|
|
For the Years Ended |
|
|||||||||||
|
Notes |
|
December 31, 2023 |
|
|
|
December 31, 2022 |
|
|
|
December 31, 2021 |
|
|||
Net cash provided by (used in) operating activities- continuing operations |
|
|
$ |
31,131 |
|
|
|
$ |
(21,835 |
) |
|
|
$ |
(24,162 |
) |
Capital expenditures for property and equipment |
|
|
|
(7,762 |
) |
|
|
|
(39,631 |
) |
|
|
|
(39,835 |
) |
Free Cash Flow |
|
|
$ |
23,369 |
|
|
|
$ |
(61,466 |
) |
|
|
$ |
(63,997 |
) |
80
The increase in Adjusted EBITDA from continuing operations for the year ended December 31, 2023, as compared to December 31, 2022 was primarily due to the implementation of adult-use sales in New Jersey and Maryland along with the reduction of general and administrative expenses.
The decrease in Adjusted EBITDA from continuing operations for year ended December 31, 2022, as compared to December 31, 2021 was primarily due to lower volume and resulting gross margin compression mainly related to challenging market dynamics in Pennsylvania.
Changes in or Adoption of Accounting Principles
New standards, amendments and interpretations adopted:
Information regarding the Company’s adoption of new accounting and reporting standards is discussed in Note 2 to the accompanying Consolidated Financial Statements included in Item 8, “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Critical Accounting Estimates and Policies
The preparation of the Company’s Consolidated Financial Statements requires management to make judgments, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and assumptions are based on historical experience and other factors that are considered relevant. Actual results may differ from these estimates.
While the Company’s significant accounting policies are described in more detail in Note 2 to the accompanying Consolidated Financial Statements included in Item 8, “Financial Statements and Supplementary Data” in this Annual Report, the accounting estimates and assumptions discussed in this section are those that the Company considers to be the most critical in the preparation of the Consolidated Financial Statements. An accounting estimate or assumption is considered critical if both (a) the nature of the estimate or assumption is material due to the levels of subjectivity and judgement involved, and (b) the impact within a reasonable range of outcomes of the estimate and assumption is material to the financial condition.
A quantitative sensitivity analysis is provided where information is available to reasonably estimate the impact and provides material information to investors.
81
Inventory
The net realizable value of inventory represents the estimated selling price in the ordinary course of business less the reasonably predictable costs of completion, disposal and transportation. The Company estimates the net realizable value of inventories, while taking into account the most reliable evidence available at each reporting date. The determination of net realizable value requires significant judgment, including consideration of factors such as shrinkage, the aging of and future demand for inventory, expected future selling price the Company expects to realize by selling the inventory, and the contractual arrangements with customers. Reserves for excess and obsolete inventory are based upon quantities on hand, projected volumes from demand forecasts and net realizable value. The future realization of these inventories may be affected by market-driven changes that may reduce future selling prices. A change to these assumptions could impact the Company’s inventory valuation and gross profit. The impact of inventory reserves is reflected in cost of sales.
Income taxes
The extent to which deferred tax assets can be recognized is based on an assessment of the probability of the Company generating future taxable income against which the deferred tax assets can be utilized. In addition, significant judgment is required in classifying transactions and assessing probable outcomes of tax positions taken, and in assessing the impact of any legal or economic limits or uncertainties in various tax jurisdictions.
Provisions for taxes are made using the best estimate of the amount expected to be paid based on a qualitative assessment of all relevant factors. The Company reviews the adequacy of these provisions at the end of the reporting period. It is possible, however, that at some future date, an additional liability could result from audits by taxing authorities. If the final outcome of these tax-related matters is different from the amounts that were initially recorded, such differences will affect the tax provisions in the period in which such determination is made.
Impairment of goodwill and intangible assets
Goodwill and intangible assets are reviewed for impairment annually and whenever there are events or changes in circumstances that indicate the carrying amount has been impaired and are tested for impairment when there are indications that an asset may be impaired. As of December 31, 2023, the carrying amount of goodwill and intangible assets was $322,783.
The Company uses an income-based approach as necessary to assess the fair values of intangible assets and its reporting units for goodwill testing purposes. Under the income approach, fair value is based on the present value of estimated cash flows. An impaired asset is written down to its estimated fair value based on the most recent information available.
Fair value is determined as the amount that would be obtained from the sale of the asset in an arm’s length transaction between knowledgeable and willing parties. Determining the value in use requires the Company to estimate expected future cash flows associated with the assets and a suitable discount rate in order to calculate present value. A number of factors, including historical results, business plans, forecasts, and market data are used to determine the fair value of the reporting unit and intangible assets.
The Company’s impairment loss calculation contains uncertainties because it requires management to make assumptions and apply judgment to qualitative factors as well as estimate future cash flows and asset fair values, including forecasting projected financial information and selecting the discount rate that reflects the risk inherent in future cash flows. If actual results are not consistent with the Company’s estimates and assumptions, the Company may be exposed to non-cash impairment losses that could be material.
Employee retention credit
The CARES Act provides for an ERC which is a refundable tax credit for businesses that continued to pay employees while shut down due to the COVID-19 pandemic, or had significant declines in gross receipts from March 13, 2020 to December 31, 2021. Eligible employers were entitled to claim the ERC on an original or adjusted employment tax return for a period within those dates. The Company has elected to account for the ERC as a government grant. There is limited grant accounting guidance within U.S. GAAP that is applicable to for-profit entities. Therefore, the Company has elected to follow the grant accounting model in International Accounting Standard ("IAS") 20, Accounting for Government Grants and Disclosure of Government Assistance. Accordingly, the Company recognized government grants for which there is a reasonable assurance of compliance with grant conditions and receipt of credits. ERC distribution in the amount of approximately $14,903 were filed by the Company. No formal determination has been made regarding the Company’s eligibility to receive ERC or if companies in the cannabis industry are generally eligible to receive ERC. There is a risk that a determination could be made that the Company, due to its operations or the nature of its business, is not eligible for the ERC distributions it has received. Additionally, there is
82
a risk that if this determination is made that the Company may not be able to repay the funds in the required timeframe and could be subject to additional penalties, which may negatively impact the Company.
Variable Interest Entity
The Company consolidates legal entities in which it holds a controlling financial interest. Determining whether it has a controlling financial interest in VIE is subject to significant judgment and estimates. There is inherent uncertainty in evaluating who has the power to direct the activities of the VIE that most significantly impact the entity's economic performance. Our considerations include, but are not limited to, voting interests of the VIE, management, service and other agreements with the VIE, involvement in the VIE’s initial design and the existence of explicit or implicit financial guarantees. Management has applied significant judgment when evaluating the facts and circumstances of the VIE (Refer to Note 3 included in Item 8, “Financial Statements and Supplementary Data”).
Emerging Growth Company Status
The Company is an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. The Company has elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that the Company (i) is no longer an emerging growth company or (ii) affirmatively and irrevocably opts out of the extended transition period provided in the JOBS Act. As a result, the Company's Consolidated Financial Statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates.
The Company will remain an emerging growth company until the earlier to occur of: (i) December 31, 2027 (a) in which the Company has total annual gross revenue of $1,235,000 or more, or (b) in which the Company is deemed to be a large accelerated filer, which means the market value of the Company's Common Stock that is held by non-affiliates exceeds $700,000 as of the last business day of the Company’s most recent second fiscal quarter; and (ii) the date on which the Company has issued more than $1,000,000 in non-convertible debt during the prior three-year period.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Market risk represents the risk of loss that may impact the Company’s financial position due to adverse changes in financial market prices and rates. The Company’s market risk exposure is primarily a result of exposure due to potential changes in inflation and interest rates. The Company does not hold financial instruments for trading purposes.
Financial Instruments and Risk Management
Financial Instruments
Financial instruments recorded at fair value are estimated by applying a fair value hierarchy, which prioritizes the inputs used to measure fair value into three levels and bases the categorization within the hierarchy upon the lowest level of input that is available and significant to the fair value measurement. The hierarchy is summarized as follows:
Risk Management
The Company’s risk exposure and the impact on the Company’s financial instruments are summarized below:
(a) Credit risk
Credit risk is the risk of financial loss to the Company if a customer or counterparty to a financial instrument fails to meet its contractual obligations. Financial instruments that potentially subject the Company to significant concentrations of credit risk consist principally of cash and cash equivalents, accounts receivable, net and notes receivable. The Company assesses the credit risk of trade receivables by evaluating the aging of trade receivables based on the invoice date. The carrying amounts of trade receivables is reduced through the use of an allowance account and the amount of the loss is recognized in the Consolidated
83
Statements of Operations and Comprehensive Income (Loss). When a trade receivable balance is considered uncollectible, it is written off against the allowance for expected credit losses.
Subsequent recoveries of amounts previously written off are credited against operating expenses in the Consolidated Statements of Operations and Comprehensive Income (Loss). The Company regularly monitors credit risk exposure and takes steps to mitigate the likelihood of these exposures resulting in actual loss. The Company had no customers whose balance is greater than 10% of total trade receivables as of December 31, 2023.
(b) Liquidity risk
The Company is exposed to liquidity risk, or the risk that the Company will not be able to meet its financial obligations as they become due. The Company manages liquidity risk through ongoing review of its capital requirements. the Company’s objective with respect to its capital management is to ensure it has sufficient cash resources to maintain its ongoing operations.
(c) Market Risk
The significant market risk exposures to which the Company is exposed are foreign currency risk and interest rate risk.
i) Foreign currency risk:
Foreign currency risk is the risk that a variation in exchange rates between the Canadian dollar and U.S. dollar and other foreign currencies will affect the Company’s operations and financial results.
The Company holds cash and cash equivalents in currencies other than its functional currency. The Company does not currently engage in currency hedging activities to limit the risks of currency fluctuations. Consequently, fluctuations in foreign currencies could have a negative impact on the profitability of the Company's operations. However, as of the year ended December 31, 2023, a 10% change in the value of the U.S. dollar compared to the Canadian dollar would not result in a material impact on unrealized foreign exchange for the Company.
ii) Interest rate risk:
Interest rate risk is the risk that future cash flows will fluctuate as a result of changes in market interest rates. In respect of financial assets, the Company’s policy is to invest excess cash at floating rates of interest in cash equivalents, in order to maintain liquidity, while achieving a satisfactory return. Fluctuations in interest rates impact the value of cash equivalents. The Company’s investments in guaranteed investment certificates bear a fixed rate and are cashable at any time prior to maturity date.
The Company does not have significant cash equivalents for the year ended December 31, 2023. The Chicago Atlantic and the Pelorus term loans have variable interest rates that are tied to the U.S. "prime rate" and SOFR. At December 31, 2023, a 10% change to each of the interest rates would result in a change to interest expense of $1,419. The remainder of the Company’s loans payable have fixed interest rates from 7.00% to 15.00% per annum. All other financial liabilities are non-interest-bearing instruments.
Item 8. Financial Statements and Supplementary Data
All information required by this item may be found on pages F-1 through F-59 of this Form 10-K.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Internal control over financial reporting is defined in Rule 13a-15(f) promulgated under the Exchange Act, as a process designed by, or under the supervision of, the Company’s principal executive officer and principal financial officer and effected by the Board, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The Company’s management, with the participation of its President and Chief Financial Officer, has evaluated the effectiveness of the Company's disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, as of
84
the end of the period covered by this Annual Report. Based upon that evaluation, management, including the President and Chief Financial Officer, determined that the Company’s disclosure controls and procedures were effective at the reasonable assurance level as of December 31, 2023.
Limitations on Effectiveness of Controls and Procedures
In designing and evaluating the Company’s disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Management’s Annual Report on Internal Controls Over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act. The Company’s management, under the supervision and with the participation of its President and Chief Financial Officer, conducted an assessment of the effectiveness of the Company’s internal control over financial reporting as of December 31, 2023. This assessment was based on criteria established in the Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) (2013 framework). This evaluation included a review of the documentation of controls, an evaluation of the design effectiveness of controls, testing of the operating effectiveness of controls and a conclusion on the outcome of the evaluation. Based on the results of its assessment, the Company’s management concluded that its internal control over financial reporting was effective as of December 31, 2023.
This Annual Report does not include an attestation report of the Company’s independent registered public accounting firm regarding the effectiveness of its internal control over financial reporting due to its exemption as an emerging growth company. Management’s report was not subject to audit by the Company’s registered public accounting firm pursuant to rules of the Securities and Exchange Commission that permit the Company to provide only management’s report in this Annual Report.
Changes in Internal Control Over Financial Reporting
There have been no changes to the Company’s internal controls over financial reporting (as defined in Rules 13a-15(f) and 15d‑15(f) under the Exchange Act) that occurred during the year ended December 31, 2023, that have materially affected, or were reasonably likely to materially affect, the Company's internal controls over financial reporting.
Item 9B. Other Information
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
85
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Information required by Item 10 of Part III will be included in the Company’s Proxy Statement relating to the Company’s 2024 Annual Meeting of Shareholders and is incorporated herein by reference.
Information regarding the Company’s Code of Business Conduct and Ethics (the “Code of Conduct”) required by this item will be contained in the Proxy Statement relating to the Annual Meeting of Shareholders and is hereby incorporated by reference. If the Company makes any substantive amendments to the Code of Conduct or grant any waiver from a provision of the Code of Conduct to any executive officer or director, it will promptly disclose the nature of the amendment or waiver on its website. The full text of the Code of Conduct is available at the Investor Relations section of the Company’s website at https://ir.terrascend.com/. The reference to the Company’s website address does not constitute incorporation by reference of the information contained at or available through the website, and you should not consider it to be a part of this Annual Report on Form 10-K.
Item 11. Executive Compensation
Information required by Item 11 of Part III will be included in the Company’s Proxy Statement relating to the Company’s 2024 Annual Meeting of Shareholders and is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Information required by Item 12 of Part III will be included in the Company’s Proxy Statement relating to the Company’s 2024 Annual Meeting of Shareholders and is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Information required by Item 13 of Part III will be included in the Company’s Proxy Statement relating to the Company’s 2024 Annual Meeting of Shareholders and is incorporated herein by reference.
Item 14. Principal Accounting Fees and Services
Information required by Item 14 of Part III will be included in the Company’s Proxy Statement relating to the Company’s 2024 Annual Meeting of Shareholders and is incorporated herein by reference.
86
PART IV
Item 15. Exhibits, Financial Statement Schedules
The accompanying Index to Consolidated Financial Statements on page F-1 of this Annual Report is provided in response to this item and is incorporated into this item by reference.
All schedules are omitted because the required information is either not present, not present in material amounts or presented within the Consolidated Financial Statements.
87
88
Exhibit Index
|
|
|
|
Incorporated By Reference |
|
|
||||||
Exhibit |
|
Exhibit Title |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed or Furnished |
|
|
|
|
|
|
|
|
|
|
|
|
|
2.1* |
|
|
10-12G |
|
000-56363 |
|
2.1 |
|
11/2/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.2* |
|
|
10-12G |
|
000-56363 |
|
2.2 |
|
11/2/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.3* |
|
|
10-12G |
|
000-56363 |
|
2.3 |
|
11/2/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.4* |
|
|
10-12G |
|
000-56363 |
|
2.4 |
|
11/2/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.5* |
|
|
10-12G |
|
000-56363 |
|
2.7 |
|
11/2/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.6 |
|
|
10-12G/A |
|
000-56363 |
|
2.8 |
|
12/22/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.7 |
|
|
8-K |
|
000-56363 |
|
10.1 |
|
03/14/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.8* |
|
Arrangement Agreement, dated August 31, 2021, by and between TerrAscend Corp. and Gage Growth Corp. |
|
10-12G |
|
000-56363 |
|
2.6 |
|
11/2/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.9 |
|
Amending Agreement, dated October 4, 2021, by and between TerrAscend Corp. and Gage Growth Corp. |
|
10-12G |
|
000-56363 |
|
2.8 |
|
11/2/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.10 |
|
|
8-K |
|
000-56363 |
|
10.2 |
|
03/14/2022 |
|
|
|
89
|
|
|
|
|
|
|
|
|
|
|
|
|
3.1 |
|
|
10-12G |
|
000-56363 |
|
3.1 |
|
11/2/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2 |
|
Articles of Amendment to the Articles of TerrAscend Corp., dated November 30, 2018. |
|
10-12G/A |
|
000-56363 |
|
3.2 |
|
12/22/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.3 |
|
Articles of Amendment to the Articles of TerrAscend Corp., dated May 22, 2020. |
|
10-12G/A |
|
000-56363 |
|
3.3 |
|
12/22/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.4 |
|
|
10-12G |
|
000-56363 |
|
3.3 |
|
11/2/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.1 |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.2 |
|
|
10-K |
|
000-56363 |
|
4.3 |
|
03/16/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.3 |
|
Form of Affiliate Gage Growth Corp. Replacement Warrants dated March, 2022. |
|
10-K |
|
000-56363 |
|
4.5 |
|
03/16/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.4 |
|
Form of Non-Affiliate Gage Growth Corp. Replacement Warrants dated March, 2022. |
|
10-K |
|
000-56363 |
|
4.6 |
|
03/16/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.5 |
|
|
10-K |
|
000-56363 |
|
4.7 |
|
03/16/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.6 |
|
|
10-K |
|
000-56363 |
|
4.1 |
|
06/29/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.1 |
|
|
10-12G |
|
000-56363 |
|
10.1 |
|
11/2/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.2* |
|
|
8-K/A |
|
000-56363 |
|
10.1 |
|
04/26/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.3* |
|
|
8-K/A |
|
000-56363 |
|
10.2 |
|
04/26/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.4* |
|
|
8-K |
|
000-56363 |
|
10.1 |
|
06/29/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.5* |
|
Form of Subscription Agreement for Equity Offering with Registered Broker-Dealer. |
|
8-K |
|
000-56363 |
|
10.2 |
|
06/29/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.6* |
|
|
8-K |
|
000-56363 |
|
10.3 |
|
06/29/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.7* |
|
|
8-K |
|
000-56363 |
|
10.4 |
|
06/29/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.8 |
|
|
10-12G |
|
000-56363 |
|
10.3 |
|
11/2/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.9 |
|
|
10-Q |
|
000-56363 |
|
10.7 |
|
08/11/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.10 |
|
|
10-K |
|
000-56363 |
|
10.4 |
|
03/16/2023 |
|
|
90
|
|
Agency Services LLC, as Administrative Agent and Collateral Agent. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.11 |
|
|
10-K |
|
000-56363 |
|
10.5 |
|
03/16/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.12 |
|
|
10-K |
|
000-56363 |
|
10.6 |
|
03/16/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.13 |
|
Amendment No. 5 to Credit Agreement, dated April 17, 2023 by and among WDB Holding PA, Inc., the Loan Parties party thereto and Acquiom Agency Services LLC as Administrative Agent and Collateral Agent. |
|
10-Q |
|
000-56363 |
|
10.7 |
|
08/10/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.14 |
|
Amendment No. 6 to Credit Agreement, dated June 22, 2023 by and among WDB Holding PA, Inc., the Loan Parties party thereto and Acquiom Agency Services LLC as Administrative Agent and Collateral Agent. |
|
10-Q |
|
000-56363 |
|
10.8 |
|
08/10/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.15 |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.16 |
|
|
10-K |
|
000-56363 |
|
10.21 |
|
03/17/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.17* |
|
|
10-Q |
|
000-56363 |
|
10.8 |
|
11/14/2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.18 |
|
|
10-K |
|
000-56363 |
|
10.9 |
|
03/16/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.19 |
|
Third Amendment to Credit Agreement and Security Agreements and Consent, dated as of June 9, 2023, |
|
10-Q |
|
000-56363 |
|
10.9 |
|
08/10/2023 |
|
|
91
|
|
among Gage Growth Corp., Gage Innovations Corp., Cookies Retail Canada Corp., WDB Holding MI, Inc., the other Borrowers party thereto, the Lenders party thereto, and Chicago Atlantic Admin, LLC, as Administrative Agent for the Lenders and Chicago Atlantic, as Collateral Agent for the Secured Parties thereto. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.20 |
|
|
10-K |
|
000-56363 |
|
10.10 |
|
03/16/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.21 |
|
Amendment No. 1, dated April 17, 2023, by and among TerrAscend NJ LLC, HMS Processing, LLC, HMS Hagerstown, LLC, HMS Health, LLC, as Borrowers, TerrAscend Corp. and TerrAscend USA, Inc., Well and Good, Inc. and WDB Holding MD, Inc., as Guarantors, and Pelorus Fund REIT, LLC, as Lender. |
|
10-Q |
|
000-56363 |
|
10.10 |
|
08/10/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.22 |
|
Amendment No. 2, dated June 22, 2023, by and among TerrAscend NJ LLC, HMS Processing, LLC, HMS Hagerstown, LLC, HMS Health, LLC, as Borrowers, TerrAscend Corp. and TerrAscend USA, Inc., Well and Good, Inc. and WDB Holding MD, Inc., as Guarantors, and Pelorus Fund REIT, LLC, as Lender. |
|
10-Q |
|
000-56363 |
|
10.11 |
|
08/10/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.23 |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.24 |
|
|
10-K |
|
000-56363 |
|
10.11 |
|
03/16/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.25 |
|
|
10-K |
|
000-56363 |
|
10.12 |
|
03/16/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.26# |
|
|
8-K |
|
000-56363 |
|
10.1 |
|
03/31/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.27# |
|
|
10-Q |
|
000-56363 |
|
10.1 |
|
11/09/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.28# |
|
Employment Agreement, dated May 23, 2022, by and between TerrAscend Corp. and Lynn Gefen. |
|
10-Q |
|
000-56363 |
|
10.2 |
|
11/09/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
92
10.29# |
|
|
10-12G |
|
000-56363 |
|
10.15 |
|
11/2/2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.30# |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.31# |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.32# |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.33# |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
21.1 |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23.1 |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
24.1 |
|
Power of Attorney (contained in the signature page to this Annual report on Form 10-K). |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
31.1 |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.2 |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32.1** |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32.2** |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.INS |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
|
|
|
|
|
|
|
|
|
|
93
* Certain confidential information has been excluded from this exhibit because it is both (i) not material and (ii) is the type of information of the Company treats as private or confidential.
# Indicates management contract or compensatory plan.
Certain schedules and exhibits have been omitted pursuant to Item 601(a)(5) of Regulation S-K. A copy of any omitted schedule or exhibit will be furnished supplementally to the Securities and Exchange Commission upon request.
** Furnished herewith and not deemed to be “filed” for purposes of Section 18 of the Exchange Act and shall not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act (whether made before or after the date of the Form 10-K), irrespective of any general incorporation language contained in such filing.
The agreements and other documents filed as exhibits to this Annual Report on Form 10-K are not intended to provide factual information or other disclosure other than with respect to the terms of the agreements or other documents themselves, and you should not rely on them for that purpose. In particular, any representations and warranties made by us in these agreements or other documents were made solely within the specific context of the relevant agreement or document and may not describe the actual state of affairs as of the date they were made or at any other time.
94
Item 16. Form 10-K Summary
Not Applicable.
95
96
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
TerrAscend Corp. |
|
|
|
|
|
Date: March 14, 2024 |
|
By: |
/s/ Ziad Ghanem |
|
|
|
Ziad Ghanem |
|
|
|
President and Chief Executive Officer |
|
|
|
(Principal Executive Officer) |
POWER OF ATTORNEY
Know all persons by these presents, that each person whose signature appears below constitutes and appoints Ziad Ghanem, Keith Stauffer and Lynn Gefen, jointly and each one of them, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place, and stead, in any and all capacities, to sign any and all amendments to this Annual Report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming that all said attorneys-in-fact and agents, or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Annual Report has been signed by the following persons in the capacities and on the dates indicated.
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
/s/ Ziad Ghanem Ziad Ghanem |
|
President and Chief Executive Officer (Principal Executive Officer) |
|
March 14, 2024 |
|
|
|
|
|
|
/s/ Keith Stauffer Keith Stauffer |
|
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
|
March 14, 2024 |
|
|
|
|
|
/s/ Jason Wild |
|
Director |
|
March 14, 2024 |
Jason Wild |
|
|
|
|
|
|
|
|
|
/s/ Ira Duarte |
|
Director |
|
March 14, 2024 |
Ira Duarte |
|
|
|
|
|
|
|
|
|
/s/ Craig Collard |
|
Director |
|
March 14, 2024 |
Craig Collard |
|
|
|
|
|
|
|
|
|
/s/ Ed Schutter |
|
Director |
|
March 14, 2024 |
Ed Schutter |
|
|
|
|
|
|
|
|
|
/s/ Kara DioGuardi |
|
Director |
|
March 14, 2024 |
Kara DioGuardi |
|
|
|
|
97
TerrAscend Corp.
Index to the Consolidated Financial Statements
|
Page |
|
|
Report of Independent Registered Public Accounting Firm (PCAOB ID: |
F-2 |
|
|
Consolidated Financial Statements: |
|
|
|
Consolidated Balance Sheets as of December 31, 2023 and December 31, 2022 |
F-3 |
|
|
F-4 |
|
|
|
F-5 |
|
|
|
F-6 |
|
|
|
F-7 |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of TerrAscend Corp.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of TerrAscend Corp. (the “Company”) as of December 31, 2023 and 2022, and the related consolidated statements of operations and comprehensive income (loss), changes in shareholders’ equity (deficit), and cash flows, for each of the years in the three-year period ended December 31, 2023, including the related notes (collectively referred to as the “consolidated financial statements”).
In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Material Uncertainty Related to Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has incurred a net loss from continuing operations and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
|
/s/
Chartered Professional Accountants, Licensed Public Accountants
|
|
|
March 14, 2024
We have served as the Company’s auditor since 2017. |
|
F-2
TerrAscend Corp.
Consolidated Balance Sheets
(Amounts expressed in thousands of United States dollars, except for per share amounts)
|
|
At |
|
|
At |
|
||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Assets |
|
|
|
|
|
|
||
Current Assets |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
|
|
$ |
|
||
Restricted cash |
|
|
|
|
|
|
||
Accounts receivable, net |
|
|
|
|
|
|
||
Investments |
|
|
|
|
|
|
||
Inventory |
|
|
|
|
|
|
||
Assets held for sale |
|
|
|
|
|
|
||
Prepaid expenses and other current assets |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Non-Current Assets |
|
|
|
|
|
|
||
Property and equipment, net |
|
|
|
|
|
|
||
Deposits |
|
|
|
|
|
|
||
Operating lease right of use assets |
|
|
|
|
|
|
||
Intangible assets, net |
|
|
|
|
|
|
||
Goodwill |
|
|
|
|
|
|
||
Other non-current assets |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Total Assets |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Liabilities and Shareholders' Equity |
|
|
|
|
|
|
||
Current Liabilities |
|
|
|
|
|
|
||
Accounts payable and accrued liabilities |
|
$ |
|
|
$ |
|
||
Deferred revenue |
|
|
|
|
|
|
||
Loans payable, current |
|
|
|
|
|
|
||
Contingent consideration payable, current |
|
|
|
|
|
|
||
Operating lease liability, current |
|
|
|
|
|
|
||
Lease obligations under finance leases, current |
|
|
|
|
|
|
||
Corporate income tax payable |
|
|
|
|
|
|
||
Other current liabilities |
|
|
|
|
|
|
||
Current liabilities from discontinued operations |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Non-Current Liabilities |
|
|
|
|
|
|
||
Loans payable, non-current |
|
|
|
|
|
|
||
Operating lease liability, non-current |
|
|
|
|
|
|
||
Lease obligations under finance leases, non-current |
|
|
|
|
|
|
||
Derivative liability |
|
|
|
|
|
|
||
Convertible debt |
|
|
|
|
|
— |
|
|
Deferred income tax liability |
|
|
|
|
|
|
||
Financing obligations |
|
|
— |
|
|
|
|
|
Liability on uncertain tax position and other long term liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Total Liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|||
Shareholders' Equity |
|
|
|
|
|
|
||
Share Capital |
|
|
|
|
|
|
||
Series A, convertible preferred stock, par value, shares authorized; |
|
|
|
|
|
|
||
Series B, convertible preferred stock, par value, shares authorized; |
|
|
|
|
|
|
||
Series C, convertible preferred stock, par value, shares authorized; and shares outstanding as of December 31, 2023 and December 31, 2022, respectively |
|
|
|
|
|
|
||
Series D, convertible preferred stock, par value, shares authorized; and shares outstanding as of December 31, 2023 and December 31, 2022, respectively |
|
|
|
|
|
|
||
Proportionate voting shares, par value, shares authorized; and shares outstanding as of December 31, 2023 and December 31, 2022, respectively |
|
|
|
|
|
|
||
Exchangeable shares, par value, shares authorized; |
|
|
|
|
|
|
||
Common shares, par value, shares authorized; |
|
|
|
|
|
|
||
Additional paid in capital |
|
|
|
|
|
|
||
Accumulated other comprehensive income |
|
|
|
|
|
|
||
Accumulated deficit |
|
|
( |
) |
|
|
( |
) |
Non-controlling interest |
|
|
( |
) |
|
|
|
|
Total Shareholders' Equity |
|
|
|
|
|
|
||
Total Liabilities and Shareholders' Equity |
|
$ |
|
|
$ |
|
||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
TerrAscend Corp.
Consolidated Statements of Operations and Comprehensive Income (Loss)
(Amounts expressed in thousands of United States dollars, except for per share amounts)
|
|
For the years ended |
|
||||||||||
|
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Revenue, net |
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|||
Cost of Sales |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|||
Gross profit |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|||
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|||
General and administrative |
|
|
|
|
|
|
|
|
|
|
|||
Amortization and depreciation |
|
|
|
|
|
|
|
|
|
|
|||
Impairment of intangible assets |
|
|
|
|
|
|
|
|
|
|
|||
Impairment of goodwill |
|
|
|
|
|
|
|
|
|
|
|||
Impairment of property and equipment |
|
|
|
|
|
|
|
|
|
|
|||
Total operating expenses |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|||
(Loss) income from operations |
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Other (income) expense |
|
|
|
|
|
|
|
|
|
|
|||
(Gain) loss from revaluation of contingent consideration |
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
Gain on extinguishment of debt |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
Gain on fair value of warrants and purchase option derivative assets |
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Gain on disposal of fixed assets |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
Finance and other expenses |
|
|
|
|
|
|
|
|
|
|
|||
Transaction and restructuring costs |
|
|
|
|
|
|
|
|
|
|
|||
(Gain) Loss on lease termination |
|
|
|
( |
) |
|
|
— |
|
|
|
|
|
Unrealized and realized foreign exchange (gain) loss |
|
|
|
( |
) |
|
|
|
|
|
|
||
Unrealized and realized loss (gain) on investments |
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
(Loss) income from continuing operations before provision for (benefit from) income taxes |
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
Provision for (benefit from) income taxes |
|
|
|
|
|
|
( |
) |
|
|
|
||
Net (loss) income from continuing operations |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Discontinued operations: |
|
|
|
|
|
|
|
|
|
|
|||
Loss from discontinued operations, net of tax |
|
|
|
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
Net (loss) income |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Foreign currency translation |
|
|
|
|
|
|
|
|
|
( |
) |
||
Comprehensive (loss) income |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Net (loss) income from continuing operations attributable to: |
|
|
|
|
|
|
|
|
|
|
|||
Common and proportionate Shareholders of the Company |
|
|
|
( |
) |
|
$ |
( |
) |
|
$ |
|
|
Non-controlling interests |
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|||
Comprehensive (loss) income attributable to: |
|
|
|
|
|
|
|
|
|
|
|||
Common and proportionate Shareholders of the Company |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|
Non-controlling interests |
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|||
Net (loss) income per share |
|
|
|
|
|
|
|
|
|
|
|||
Net (loss) income per share - basic: |
|
|
|
|
|
|
|
|
|
|
|||
Continuing operations |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|
Discontinued operations |
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Net (loss) income per share - basic |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|
Weighted average number of outstanding common and proportionate voting shares |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|||
Net (loss) income per share - diluted: |
|
|
|
|
|
|
|
|
|
|
|||
Continuing operations |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|
Discontinued operations |
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Net (loss) income per share - diluted |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|
Weighted average number of outstanding common and proportionate voting shares, assuming dilution |
|
|
|
|
|
|
|
|
|
|
|||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
TerrAscend Corp.
Consolidated Statements of Changes in Shareholders’ Equity (Deficit)
(Amounts expressed in thousands of United States dollars, except for per share amounts)
|
|
Number of Shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
Convertible Preferred Stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
|
|
Common Shares |
|
Exchangeable Shares |
|
Proportionate Voting Shares |
|
Series A |
|
Series B |
|
Series C |
|
Common Shares Equivalent |
|
|
|
Additional paid in capital |
|
|
|
Accumulated other comprehensive income (loss) |
|
|
|
Accumulated deficit |
|
|
|
Non-controlling interest |
|
|
Total |
|
||||||||||||||||
Balance at December 31, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
$ |
|
|
|
$ |
( |
) |
|
|
$ |
( |
) |
|
|
$ |
|
|
$ |
( |
) |
||||||||||||
Shares issued - stock options, warrant and RSU |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
|||||||||
Shares issued - acquisitions |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
||||||||
Shares issued - liability settlement |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
||||||||
Private placement net of share issuance costs |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
||||||||
Shares issued - conversion |
|
|
|
|
— |
|
|
( |
) |
|
( |
) |
|
( |
) |
|
( |
) |
|
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
||||||
Share-based compensation expense |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
||||||
Options and warrants expired/forfeited |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
( |
) |
|
|
|
— |
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|||||
Conversion of convertible debt |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
||||||||
Investment in NJ Partnership |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
( |
) |
|
|
|
— |
|
|
|
|
— |
|
|
|
|
( |
) |
|
|
( |
) |
||||
Capital distributions |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
( |
) |
|
|
( |
) |
||||
Net income for the year |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Foreign currency translation |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
— |
|
|
|
|
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
||||||
Balance at December 31, 2021 |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
|
$ |
|
|
$ |
|
||||||||||||||
Shares issued - stock options, warrant and RSU |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
||||||||
Shares, options and warrants issued - acquisitions |
|
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
|||||||||
Shares issued - liability settlement |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
||||||||
Shares issued - conversion |
|
|
|
|
— |
|
|
— |
|
|
( |
) |
|
( |
) |
|
( |
) |
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|||||
Shares issued- Canopy USA arrangement |
|
|
— |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
||||||||
Share-based compensation expense |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
||||||
Options and warrants expired/forfeited |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
( |
) |
|
|
|
— |
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|||||
Capital distributions |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
( |
) |
|
|
( |
) |
||||
Net loss for the year |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
( |
) |
|
|
|
|
|
|
( |
) |
|||||
Foreign currency translation |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
— |
|
|
|
|
( |
) |
|
|
|
— |
|
|
|
|
— |
|
|
|
( |
) |
||||
Balance at December 31, 2022 |
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
|
|
|
|
— |
|
|
( |
) |
|
— |
|
|
|
|
$ |
|
||||||||||
Shares issued - stock options, warrant and RSU |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||||
Shares, options and warrants issued - acquisitions |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||||
Shares, options and warrants issued - legal settlement |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||||
Warrants issued for services performed |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||
Shares issued - conversion |
|
|
|
|
( |
) |
|
— |
|
|
( |
) |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
Private placement net of share issuance costs |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||||
Share-based compensation expense |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||
Options and warrants expired/forfeited |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
( |
) |
|
— |
|
|
— |
|
|
— |
|
|
|
|
— |
|
|
— |
|
|
|
— |
|
|
Capital distributions |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
( |
) |
|
|
( |
) |
Acquisition of non-controlling interest |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
( |
) |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
( |
) |
|
|
( |
) |
Net loss for the year |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
( |
) |
|
— |
|
|
|
|
|
( |
) |
|
Foreign currency translation |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
( |
) |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
( |
) |
Balance at December 31, 2023 |
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
|
$ |
( |
) |
|
$ |
|
||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
TerrAscend Corp.
Consolidated Statements of Cash Flows
(Amounts expressed in thousands of United States dollars, except for per share amounts)
|
For the Twelve Months Ended |
|
|||||||||
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Operating activities |
|
|
|
|
|
|
|
|
|||
Net (loss) income from continuing operations |
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|
Adjustments to reconcile net (loss) income to net cash provided by (used in) operating activities |
|
|
|
|
|
|
|
|
|||
Non-cash adjustments of inventory |
|
|
|
|
|
|
|
|
|||
Accretion expense |
|
|
|
|
|
|
|
|
|||
Depreciation of property and equipment and amortization of intangible assets |
|
|
|
|
|
|
|
|
|||
Amortization of operating right-of-use assets |
|
|
|
|
|
|
|
|
|||
Share-based compensation |
|
|
|
|
|
|
|
|
|||
Deferred income tax expense |
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Gain on fair value of warrants and purchase option derivative |
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Gain on disposal of fixed assets |
|
( |
) |
|
|
— |
|
|
|
— |
|
(Gain) loss from revaluation of contingent consideration |
|
( |
) |
|
|
( |
) |
|
|
|
|
Impairment of goodwill and intangible assets |
|
|
|
|
|
|
|
|
|||
Impairment of property and equipment |
|
|
|
|
|
|
|
|
|||
(Gain) loss on derecognition of right of use assets and lease termination |
|
( |
) |
|
|
|
|
|
|
||
Release of indemnification asset |
|
— |
|
|
|
|
|
|
|
||
Forgiveness of loan principal and interest |
|
— |
|
|
|
— |
|
|
|
( |
) |
Bad debt expense |
|
— |
|
|
|
|
|
|
— |
|
|
Employee Retention Credits recorded in other income |
|
— |
|
|
|
( |
) |
|
|
— |
|
Gain on extinguishment of debt |
|
— |
|
|
|
( |
) |
|
|
— |
|
Debt modification fees expensed |
|
— |
|
|
|
|
|
|
— |
|
|
Unrealized and realized foreign exchange (gain) loss |
|
( |
) |
|
|
|
|
|
|
||
Unrealized and realized loss (gain) on investments |
|
|
|
|
( |
) |
|
|
( |
) |
|
Changes in operating assets and liabilities |
|
|
|
|
|
|
|
|
|||
Receivables |
|
( |
) |
|
|
|
|
|
( |
) |
|
Inventory |
|
( |
) |
|
|
|
|
|
( |
) |
|
Prepaid expense and other current assets |
|
|
|
|
|
|
|
( |
) |
||
Deposits |
|
|
|
|
|
|
|
— |
|
||
Other assets |
|
|
|
|
|
|
|
( |
) |
||
Accounts payable and accrued liabilities and other payables |
|
|
|
|
( |
) |
|
|
|
||
Operating lease liability |
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Other liability |
|
( |
) |
|
|
( |
) |
|
|
|
|
Uncertain tax position liabilities |
|
|
|
|
|
|
|
( |
) |
||
Contingent consideration payable |
|
— |
|
|
|
( |
) |
|
|
( |
) |
Corporate income tax payable |
|
( |
) |
|
|
|
|
|
( |
) |
|
Deferred revenue |
|
|
|
|
|
|
|
|
|||
Net cash provided by (used in) operating activities- continuing operations |
|
|
|
|
( |
) |
|
|
( |
) |
|
Net cash used in operating activities - discontinued operations |
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Net cash provided by (used in) operating activities |
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|||
Investing activities |
|
|
|
|
|
|
|
|
|||
Investment in property and equipment |
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Investment in intangible assets |
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Principal payments received on lease receivable |
|
— |
|
|
|
|
|
|
|
||
Distribution of earnings from associates |
|
— |
|
|
|
— |
|
|
|
|
|
Investment in NJ partnership |
|
— |
|
|
|
— |
|
|
|
( |
) |
Deposits for business acquisition |
|
— |
|
|
|
( |
) |
|
|
— |
|
Success fees related to ATC and other investment |
|
( |
) |
|
|
— |
|
|
|
— |
|
Payment for land contracts |
|
( |
) |
|
|
( |
) |
|
|
— |
|
Cash portion of consideration (paid in) received acquisitions, net of cash of acquired |
|
( |
) |
|
|
|
|
|
( |
) |
|
Net cash used in investing activities - continuing operations |
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Net cash provided by (used in) investing activities - discontinued operations |
|
|
|
|
( |
) |
|
|
( |
) |
|
Net cash used in investing activities |
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||
Financing activities |
|
|
|
|
|
|
|
|
|||
Transfer of Employee Retention Credit |
|
|
|
|
— |
|
|
|
— |
|
|
Proceeds from loan payable, net of transaction costs |
|
|
|
|
|
|
|
|
|||
Proceeds from options and warrants exercised |
|
|
|
|
|
|
|
|
|||
Loan principal paid |
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Loan amendment fee paid and prepayment premium paid |
|
( |
) |
|
|
( |
) |
|
|
— |
|
Tax distributions to NJ partners |
|
|
|
|
( |
) |
|
|
— |
|
|
Capital distributions paid to non-controlling interests |
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Payments of contingent consideration |
|
— |
|
|
|
( |
) |
|
|
( |
) |
Proceeds from private placement, net of share issuance costs |
|
|
|
|
— |
|
|
|
|
||
Payments made for financing obligations and finance lease |
|
( |
) |
|
|
( |
) |
|
|
— |
|
Net cash (used in) provided by financing activities- continuing operations |
|
( |
) |
|
|
|
|
|
|
||
Net cash used in financing activities- discontinued operations |
|
( |
) |
|
|
— |
|
|
|
— |
|
Net cash (used in) provided by financing activities |
|
( |
) |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|||
Net (decrease) increase in cash and cash equivalents and restricted cash during the year |
|
( |
) |
|
|
( |
) |
|
|
|
|
Net effects of foreign exchange |
|
( |
) |
|
|
( |
) |
|
|
|
|
Cash and cash equivalents and restricted cash, beginning of the year |
|
|
|
|
|
|
|
|
|||
Cash and cash equivalents and restricted cash, end of the year |
$ |
|
|
$ |
|
|
$ |
|
|||
|
|
|
|
|
|
|
|
|
|||
Supplemental disclosure with respect to cash flows |
|
|
|
|
|
|
|
|
|||
Income taxes (refund received) paid |
$ |
( |
) |
|
$ |
|
|
$ |
|
||
Interest paid |
$ |
|
|
$ |
|
|
$ |
|
|||
Lease termination fee paid |
$ |
|
|
$ |
|
|
$ |
— |
|
||
Non-cash transactions |
|
|
|
|
|
|
|
|
|||
Equity and warrant liability issued as consideration for acquisition |
$ |
|
|
$ |
|
|
$ |
|
|||
Shares issued for Canopy USA arrangement |
$ |
— |
|
|
$ |
|
|
$ |
— |
|
|
Warrant issued as consideration for services |
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|
Promissory note issued as consideration for acquisitions |
$ |
|
|
$ |
|
|
$ |
|
|||
Shares issued for legal and liability settlement |
$ |
|
|
$ |
|
|
$ |
— |
|
||
Accrued capital purchases |
$ |
|
|
$ |
|
|
$ |
|
|||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
TERRASCEND CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
TerrAscend Corp. (the "Issuer") was incorporated under the Business Corporations Act (Ontario) on
The Company operates under one operating segment, which is the cultivation, production and sale of cannabis products.
The Company owns a portfolio of operating businesses, including:
The common shares in the capital of the Company ("Common Shares") commenced trading on the Canadian Securities Exchange ("CSE") on May 3, 2017 under the ticker symbol "TER" and continued trading on the CSE until the listing of the Common Shares on the Toronto Stock Exchange (the "TSX"). Effective July 4, 2023, the Common Shares commenced trading on the TSX under the ticker symbol "TSND". The Common Shares commenced trading on OTCQX on October 22, 2018 under the ticker symbol "TRSSF", which was subsequently changed to "TSNDF", effective July 6, 2023. The Company’s registered office is located at 77 City Centre Drive, Suite 501, Mississauga, Ontario, L5B 1M5, Canada.
2. Summary of significant accounting policies
(a) Basis of presentation and measurement and going concern
These consolidated financial statements as of December 31, 2023 and December 31, 2022 and for the years ended December 31, 2023, December 31, 2022, and December 31, 2021 (the “Consolidated Financial Statements”) of the Company were prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”).
The accompanying Consolidated Financial Statements have been prepared on the going concern basis, under the historical cost convention, except for certain financial instruments that are measured at fair value as described herein. As of December 31, 2023, the Company had an accumulated deficit of $
F-7
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
The aforementioned indicators raise substantial doubt about the Company's ability to continue as a going concern for at least one year from the issuance of these Consolidated Financial Statements. The Company believes this concern is mitigated by steps it has taken, or intends to take to improve its operations and cash position, including: (i) identifying access to future capital required to meet the Company’s on-going obligations, (ii) improved cashflow growth from the Company's consolidated operations, particularly TerrAscend's operations in New Jersey and most recently Maryland with conversion to adult-use sales, and (iii) various cost and efficiency improvements. The Consolidated Financial Statements do not include any adjustments relating to the recoverability and classification of asset carrying amounts or the amounts of and classification of liabilities that may result should the Company be unable to continue as a going concern.
(b) Functional and presentation currency
All operations in the United States have a functional currency of the U.S dollar ("USD"). Canadian operations have a functional currency of Canadian dollars (“CAD”). The Company’s presentation currency is in USD. All amounts are presented in USD unless otherwise specified. References to CAD are to Canadian dollars.
(c) Basis of consolidation
These Consolidated Financial Statements include the financial information of the Company. The Company consolidates legal entities in which it holds a controlling financial interest. The Company has a two-tier consolidation model: one focused on voting rights (the voting interest model) and the second focused on a qualitative analysis of power over significant activities and exposure to potentially significant losses or benefits (the variable interest model). All entities are first evaluated to determine whether they are variable interest entities (“VIE”). If an entity is determined not to be a VIE, it is assessed on the basis of voting and other decision-making rights under the voting interest model ("VOE").
Voting Interest Entities
A VOE is an entity in which (i) the total equity investment at risk is deemed sufficient to absorb the expected losses of the entity, (ii) the at-risk equity holders, as a group, have all of the characteristics of a controlling financial interest and (iii) the entity is structured with substantive voting rights. The Company consolidates its Canadian operations under a VOE model based on the controlling financial interest obtained through Common Shares with substantive voting rights.
Variable Interest Entities
A VIE is an entity that lacks one or more characteristics of a controlling financial interest defined under the voting interest model. The Company consolidates VIE when it has a variable interest that provide it with (i) the power to direct the activities of a VIE that most significantly impact the VIE’s economic performance (power) and (ii) the obligation to absorb losses of the VIE that potentially could be significant to the VIE or the right to receive benefits from the VIE that potentially could be significant to the VIE (benefits).
The accounts of the subsidiaries are prepared for the same reporting period using consistent accounting policies. For further information on VIEs, see Note 3.
All intercompany balances and transactions were eliminated on consolidation.
(d) Cash, cash equivalents and restricted cash
Cash and cash equivalents include cash on hand at retail locations, demand deposits with financial institutions and other short-term, highly liquid investments with original maturities of three months or less that are readily convertible to known amounts of cash and subject to an insignificant risk of change in value. Cash held in money market investments are carried at fair value, cash held in financial institutions and cash held at retail locations have carrying values that approximate fair value. Restricted cash consists of cash held with financial institutions which are subject to certain withdrawal restrictions.
F-8
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
(e) Accounts Receivable
Accounts receivable are recorded net of current expected credit losses. The Company estimates current expected credit losses based on existing contractual payment terms, actual payment patterns of its customers and individual customer circumstances.
(f) Inventory
Inventories of harvested and purchased finished goods as well as packaging materials are valued at the lower of cost or net realizable value. Net realizable value is determined as the estimated selling price in the ordinary course of business less the reasonably predictable costs of completion, disposal and transportation. The direct and indirect costs of inventory include materials, labor and depreciation expense on property and equipment involved in packaging, labeling and inspection. Inventories are generally maintained with the average cost method. Amortization of acquired cannabis production licenses as well as royalties paid relating to the production of inventory are also considered to be indirect costs of inventory. All direct and indirect costs related to inventory are capitalized as they are incurred and they are subsequently recorded within cost of sales on the Consolidated Statements of Operations and Comprehensive Income (Loss) at the time cannabis is sold.
Products for resale and supplies and consumables are valued at the lower of cost or net realizable value. The Company reviews inventory for obsolete, redundant, and slow-moving goods, and any such inventories are written down to net realizable value.
(g) Property and equipment and long-lived assets held for sale
Property and equipment is measured at cost, including capitalized borrowing costs, less accumulated depreciation and impairment losses. Ordinary repairs and maintenance are expensed as incurred.
Buildings and improvements |
|
Land |
Not depreciated |
Machinery & equipment |
|
Office furniture & production equipment |
|
Right of use assets |
|
Assets in process |
Not depreciated |
Assets in process are transferred to the appropriate asset type when available for use and depreciation of the assets commences at that point.
The Company classifies assets and liabilities (the "disposal group") as held for sale in the period when all of the relevant criteria to be classified as held for sale are met. Long-lived assets held for sale are recorded at the lower of its carrying value or fair value less costs to sell. Any loss resulting from the measurement is recognized in the period during which the held for sale criteria is met. The Company discontinues depreciation on these assets.
An asset’s residual value, useful life and depreciation method are reviewed annually, or when events or circumstances indicate that the current estimate or depreciation method are no longer applicable. Changes are adjusted prospectively if appropriate. Gains and losses on disposal of an asset are determined by comparing the proceeds from disposal with the carrying amount of the items and are recognized in the Consolidated Statements of Operations and Comprehensive Income (Loss).
The Company evaluates the recoverability of property and equipment whenever events or changes in circumstances indicate that the carrying value of the asset or asset group may not be recoverable. See – Impairment of long-lived assets information within this note for detailed information on the Company’s impairment assessment of its property and equipment.
The Company capitalizes interest and borrowing costs on significant qualifying capital construction projects. Upon the asset becoming available for use, capitalization of borrowing costs ceases, and depreciation commences on a straight-line basis over the estimated useful life of the related asset.
(h) Leases
Leases are classified as operating or finance leases based on the terms of the lease agreement and certain characteristics of the identified assets. The majority of the Company’s leases are operating leases used primarily for corporate offices, retail
F-9
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
dispensaries, and cultivation and manufacturing facilities. The operating lease periods range from
The Company’s leases include fixed payments, as well as in some cases, scheduled base rent increases over the term of the lease. Certain leases require variable payments of common area maintenance, operating expenses, and real estate taxes applicable to the property. Variable payments are excluded from the measurements of lease liabilities and are expensed as incurred. Any tenant improvement allowances received from the lessor are recorded as a reduction to rent expense over the term of the lease. None of the Company’s lease agreements contain residual value guarantees or material restrictive covenants.
The Company determines if an arrangement is a lease at the inception of the contract. Lease liabilities are recognized at the commencement date based on the present value of lease payments over the lease term for those arrangements where there is an identified asset and the contract conveys the right to control its use. The right-of-use (“ROU”) asset is measured at the initial amount of the lease liability, adjusted for lease payments made at or before the lease commencement date, and initial direct costs. For operating leases, right-of-use assets are reduced over the lease term by the straight-line expense recognized, less the amount of accretion of the lease liability determined using the effective interest rate method. Finance leases are included in property and equipment in the Company's Consolidated Balance Sheets.
Operating lease expense is recognized on a straight-line basis over the term of the lease and is included in cost of sales and general and administrative expense in the Company’s Consolidated Statements of Operations and Comprehensive Income (Loss). Finance lease cost includes amortization, which is recognized on a straight-line basis over the expected life of the lease asset, and interest expense, which is recognized following an effective interest rate method and is included in finance and other expenses in the Company’s Consolidated Statements of Operations and Comprehensive Income (Loss).
The majority of the Company’s leases do not provide an implicit rate that can be easily determined. Therefore, the Company applies its incremental borrowing rate to the lease based on the information available at the commencement date (refer to Note 11 included in Item 8, "Financial Statements and Supplementary Data").
The Company evaluates its ROU assets for impairment consistent with its impairment of long-lived assets. See – Impairment of long-lived assets information within this note for detailed information on the Company’s impairment assessment of its right-of-use assets.
In some instances, the Company subleases excess office space to third-party tenants. The Company, as sublessor, continues to account for the head lease. If the lease cost for the term of the sublease exceeds the Company’s anticipated sublease income for the same period, this indicates that the ROU asset associated with the head lease should be assessed for impairment under the long-lived asset impairment provisions. Sublease income is included in Finance (expense) income in the Company’s Consolidated Statements of Operations and Comprehensive Income (Loss).
The Company accounts for non-lease and lease components to which they relate as a single lease component. Additionally, the Company recognized lease payments under short-term leases with an initial term of twelve months or less, as well as low value assets, as an expense on a straight-line basis over the lease term without recognizing the lease liability and ROU asset.
(i) Goodwill
Goodwill is recorded at the time of acquisition and represents the excess of the aggregate consideration paid for an acquisition over the fair value of the net tangible and intangible assets acquired. Goodwill is not subject to amortization and is tested for impairment on an annual basis or more frequently if events or changes in circumstances indicate that they might be impaired.
F-10
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
See – Impairment of goodwill and intangible assets information within this note for detailed information on the Company’s impairment assessment of its goodwill and intangible assets.
(j) Intangible assets
Intangible assets are recorded at cost less accumulated amortization and impairment losses, if any. Intangible assets acquired in a business combination are measured at fair value at the acquisition date. Amortization is provided on a straight-line basis over the assets’ estimated useful lives, which do not exceed the contractual period, if any. The estimated useful lives, residual values and amortization methods are reviewed annually and any changes in estimates are accounted for prospectively.
Brand intangibles- indefinite lives |
Indefinite useful lives |
Brand intangibles- definite lives |
|
Software |
|
Licenses |
|
Non-compete agreements |
Licenses relating to cultivation and dispensaries are amortized using a useful life consistent with the property and equipment to which they relate.
Intangible assets that have indefinite useful lives, which include brand names, are not subject to amortization but the carrying value is tested for impairment on an annual basis or more frequently if events or changes in circumstances indicate that they may be impaired. See – Impairment of long-lived assets information within this note for detailed information on the Company’s impairment assessment of its goodwill and intangible assets.
(k) Impairment of indefinite lived intangible assets and goodwill
The Company operates as
Goodwill is reviewed for impairment annually and whenever there are events or changes in circumstances that indicate the carrying amount has been impaired. The Company has the option to first assess qualitative factors to determine whether a quantitative goodwill impairment test is necessary. If the Company concludes it is more likely than not that the fair value of a reporting unit exceeds its carrying value, a quantitative fair value test is performed. If the carrying value of the reporting unit exceeds the estimated fair value, a goodwill impairment charge is recorded.
Indefinite lived intangible assets are tested for impairment annually or more frequently if events or changes in circumstances between annual tests indicate that the asset may be impaired. Impairment losses on indefinite lived intangible assets are recognized based on a comparison of the fair value of the asset to its carrying value.
(l) Impairment of long-lived assets and definite lived intangible assets
The Company evaluates the recoverability of long-lived assets, including property and equipment, ROU assets, and definite lived intangible assets, based on whether events or changes in circumstances indicate that the carrying value of the asset, or asset group, may not be recoverable.
When the Company determines that the carrying value of the long-lived asset may not be recoverable based upon the existence of one or more indicators, the assets are assessed for impairment based on the estimate of future undiscounted cash flows expected to result from the use of the asset and its eventual disposition. If the carrying value of an asset exceeds its estimated future undiscounted cash flows, an impairment loss is recorded for the excess of the asset’s carrying value over its estimated fair value.
(m) Revenue recognition
Revenue is recognized by the Company in accordance with ASU 2014-09 Revenue from Contracts with Customers (Topic 606). The standard requires sales to be recognized in a manner that depicts the transfer of promised goods or services to a
F-11
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
customer and at an amount that reflects the consideration expected to be received in exchange for transferring those goods or services. This is achieved by applying the following five steps: i) identify the contract with a customer; ii) identify the performance obligations in the contract; iii) determine the transaction price; iv) allocate the transaction price to the performance obligations in the contract; and v) recognize sales when (or as) the entity satisfies a performance obligation.
Revenues consist of wholesale and retail sales, which are recognized when control of the goods has transferred to the purchaser and the collectability is reasonably assured. This is generally when goods have been delivered, which is also when the performance obligations have been fulfilled under the terms of the related sales contract. Revenue from retail sales of cannabis to customers for a fixed price is recognized when the Company transfers control of the goods to the customer at the point of sale and the customer has accepted and paid for the goods. Revenue for wholesale sales for a fixed price is recognized upon delivery to the customer. Sales are recorded net of returns and discounts and incentives, but inclusive of freight. Payment is typically due upon transferring the goods to the customer or within a specified time period permitted under the Company’s credit policy. All shipping and handling activities are performed before the customers obtain control of products and are accounted for as cost of sales.
From time to time, the Company enters into sales agreements with suppliers pursuant to which it also purchases inventory. As part of the five-step revenue model, the Company assesses whether instances of bulk sales made to suppliers of goods have commercial substance and should be recognized as revenue, or whether they should be assessed under Accounting Standards Codification ("ASC") 845, Nonmonetary Transactions.
Local authorities will often impose excise or cultivation taxes on the sale or production of cannabis products. Excise and cultivation taxes are effectively a production tax which become payable when a cannabis product is delivered to the customer and are not directly related to the value of sales. The Company has made a policy election to exclude from the measurement of transaction price all taxes assessed by a governmental authority that are both imposed on and concurrent with the specific revenue-producing transaction and collected by the company from a customer. Therefore, the net revenue on the Consolidated Statements of Operations and Comprehensive Income (Loss) is netted for any excise or cultivation taxes.
(n) Business combinations
The Company accounts for business combinations using the acquisition method when control is obtained by the Company (see Note 2(c)). The Company measures the consideration transferred, the assets acquired, and the liabilities assumed in a business combination at their acquisition-date fair values. Acquisition related costs are recognized as expenses in the periods in which the costs are incurred, and the services are received, except for the costs to issue debt or equity securities which are recognized according to specific requirements. The excess of the consideration transferred to obtain control, over the net of the acquisition-date amounts of the identifiable assets acquired and the liabilities assumed, is recognized as goodwill as of the acquisition date.
Contingent consideration for a business combination is measured at its acquisition-date fair value and included as part of the consideration transferred in a business combination. Contingent consideration that is classified as a liability is measured at subsequent reporting dates at fair value with the corresponding gain or loss being recognized in profit or loss.
If the acquiree’s former owners contractually indemnify the Company for a particular uncertainty, an indemnification asset is recognized on a basis that matches the indemnified item, subject to the contractual provisions or any collectability considerations.
F-12
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
(o) Investments
The majority of the Company's investments are initially recorded at cost. Management assesses investments for impairment on an annual basis, or when events or changes in circumstances indicate that the carrying value of the investment may not be recoverable.
(p) Non-controlling interests
Non-controlling interests (“NCI”) represents equity interests owned by outside parties. NCI is initially measured at fair value as of the acquisition date (refer to Note 2z(viii)).
(q) Income taxes
Income tax expense, consisting of current and deferred tax expense, is recognized in the Consolidated Statements of Operations and Comprehensive Income (Loss). Current tax expense is the expected tax payable on the taxable income for the year, using tax rates enacted at period-end, adjusted for amendments to tax payable with regard to previous years. Deferred tax assets and liabilities and the related deferred income tax expense or recovery are recognized for deferred tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis. Deferred tax assets and liabilities are measured using the enacted tax rates expected to apply when the asset is realized, or the liability settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income (loss) in the period that enactment occurs. A deferred tax asset is recognized to the extent that it is probable that future taxable profits will be available against which the asset can be utilized. To the extent that the Company does not consider it more likely than not that a deferred tax asset will be recovered, the deferred tax asset is reduced. Deferred tax assets and liabilities are offset when there is a legally enforceable right to set off current tax assets against current tax liabilities and when they relate to income taxes levied by the same taxation authority and the Company intends to settle its current tax assets and liabilities on a net basis.
The Internal Revenue Service (the "IRS") has taken the position that cannabis companies are subject to the limits of Section 280E of the Internal Revenue Code of 1986, as amended (the "Code"), under which they are only allowed to deduct expenses directly related to the cost of producing the products or cost of production. The Company has taken the position that it does not owe taxes attributable to the application of Section 280E the Code. This position is treated as an unrecognized tax benefit, and is recorded on the Consolidated Balance Sheets as a liability on uncertain tax position and other long term liabilities.
(r) Share capital
Common Shares
Common Shares are classified as equity. The proceeds from the exercise of stock options and warrants are recorded as share capital. Incremental costs directly attributable to the issuance of Common Shares are recognized as a deduction from equity.
Equity units
Equity units are comprised of Common Shares and one-half warrants. Warrants issued during the year are classified as liabilities. The proceeds are allocated first to warrants based on its fair value, measured using the Black-Scholes Option Pricing Model ("Black-Scholes Model"), and the residual was allocated to Common Shares.
(s) Share-based compensation
The Company has a stock option plan in place (the "Stock Option Plan"). The Company measures equity settled share-based payments based on their fair value at the grant date and recognizes compensation expense on a straight-line basis over the vesting period. Fair value is measured using the Black-Scholes Model. In estimating fair value, management is required to make certain assumptions and estimates such as the expected life of units, volatility of the Company’s future share price, risk free rates, expected forfeiture and future dividend yields at the initial grant date. Changes in assumptions used to estimate fair value could result in materially different results. Expected forfeitures are estimated at the date of grant, based on historical trends of actual option forfeitures, and subsequently adjusted if further information indicates actual forfeitures may vary from the original estimate. Any revisions are recognized in the Consolidated Statements of Operations and Comprehensive Income (Loss) such that the cumulative expense reflects the revised estimate. If the actual forfeiture rate is materially different from
F-13
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
management’s estimates, the stock-based compensation expense could be significantly different from what the Company has recorded in the current period.
Upon exercise of stock options and warrants that are classified as equity, any historical fair value in the warrants and share-based compensation reserve is allocated to additional paid in capital. Amounts recorded for expired unexercised stock options and warrants are transferred to deficit in the year of expiration.
The fair value of restricted share units is based on the closing price of the Company’s stock as of the grant date. Compensation expense is recognized on a straight-line basis, by amortizing the grant date fair value over the vesting period.
(t) Convertible instruments
The Company evaluates and accounts for conversion options embedded in convertible instruments in accordance with ASC 815, Derivatives and Hedging Activities.
The Company issued convertible debentures with detachable share purchase warrants at various times to raise capital to expand its business and support general corporate needs. The convertible instruments also included embedded derivatives in the form of conversion features and put options. Management evaluated the convertible debentures to determine the proper accounting and whether the embedded derivatives required bifurcation from the host instrument.
In accordance with ASC 815, Derivatives and Hedging, the conversion option was bifurcated from the host instrument as the instrument's strike price is denominated in a currency other than the functional currency of the Issuer. The proceeds are allocated first to the conversion option based on its fair value, measured using the Black-Scholes model, and the residual was allocated to the host instrument and recorded as convertible debt at amortized cost.
(u) Convertible preferred stock and detachable warrants
The Company evaluates convertible preferred stock in accordance with ASC 470-20-35-7, Debt with Conversion and Other Options. The preferred shares in the capital of the Company ("Preferred Shares") are convertible into Common Shares at a conversion ratio of
Included in the issuance were detachable warrants to purchase Preferred Shares. The detachable purchase warrants were evaluated for equity or liability classification and were determined to meet liability classification. The warrants are legally detachable and separately exercisable from the Preferred Shares.
(v) Warrant liability
The Company may issue Common Share warrants with debt, equity or as a standalone financing instrument that is recorded as either liabilities or equity in accordance with the respective accounting guidance. Warrants recorded as equity are recorded at their relative fair value determined at the issuance date and remeasurement is not required. Warrants recorded as liabilities are recorded at their fair value, within warrant liability on the Consolidated Balance Sheets, and remeasured on each reporting date with changes recorded in the Company's Consolidated Statements of Operations and Comprehensive Income (Loss).
(w) Embedded derivative liabilities
The Company evaluates its financial instruments to determine if those instruments or any embedded components of those instruments qualify as derivatives that need to be separately accounted for in accordance with ASC 815, Derivatives and Hedging. Embedded derivatives satisfying certain criteria are recorded at fair value at issuance and marked-to-market at each balance sheet date with the change in the fair value recorded as income or expense. In addition, upon the occurrence of an event that requires the derivative liability to be reclassified to equity, the derivative liability is revalued to fair value at that date.
(x) (Loss) earnings per share
The Company presents basic and diluted (loss) earnings per share data for its ordinary shares. Basic (loss) earnings per share is calculated using the treasury stock method, by dividing the (loss) income attributable to holders of Common Shares and proportionate voting shares in the capital of the Company ("Proportionate Voting Shares") by the weighted average number of
F-14
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
Common Shares and Proportionate Voting Shares outstanding during the period. Contingently issuable shares (including shares held in escrow) are not considered outstanding Common Shares and consequently are not included in the (loss) earnings per share calculations. The Company has the following categories of potentially dilutive common share equivalents: RSUs, stock options, warrants, Preferred Shares, exchangeable shares in the capital of the Company ("Exchangeable Shares") and convertible debentures.
In order to determine diluted (loss) earnings per share, it is assumed that any proceeds from the exercise of dilutive instruments would be used to repurchase Common Shares at the average market price during the period. The Company also considers all outstanding convertible securities, such as the Preferred Shares, convertible debentures, and outstanding Exchangeable Shares as if such instruments were converted into Common Shares.
Diluted (loss) earnings per share is determined by adjusting the (loss) income attributable to common shareholders and the weighted average number of Common Shares and Proportionate Voting Shares outstanding, adjusted for the effects of all dilutive potential Common Shares and Proportionate Voting Shares. Proportionate Voting Shares are converted to their Common Share equivalent of one thousand Common Shares for every one Proportionate Voting Share for the purposes of calculating basic and diluted (loss) earnings per share. In a period of losses, all of the potentially dilutive Common Share equivalents are excluded in the determination of dilutive net loss per share because their effect is antidilutive. During the years ended December 31, 2023 and 2022,
(y) Discontinued operations
The Company deems it appropriate to classify a part of the business as discontinued operations if the related disposal group meets all of the following criteria: (i) the disposal group is a component of the Company, (ii) the component meets the held-for-sale criteria, and (iii) the disposal of the component represents a strategic shift that has a major effect on the Company's operations and financial results. A disposal group that represents a strategic shift to the Company is reflected as discontinued operations on the Consolidated Statements of Operations and Comprehensive Income (Loss) and prior periods are recast to reflect the earnings or losses as income from discontinued operations.
TerrAscend Canada is a cannabis retailer in Ontario, Canada. TerrAscend Canada operates the Company's a majority-owned Cookies Canada dispensary in Toronto, Ontario, Canada. TerrAscend Canada was previously a Licensed Producer (as such term is defined in the Cannabis Act) of cannabis until the Company commenced an optimization of its operations in Canada, whereby the Company reduced its manufacturing footprint in order to focus on its Canadian retail business, as well as monetize its intellectual property portfolio in Canada. The Company ceased operations at TerrAscend Canada's manufacturing facility during the three months ended December 31, 2022.
All prior year amounts from discontinued operations have been reclassified for consistency with the current year presentation.
(z) Use of significant estimates and judgments
The preparation of the Company’s Consolidated Financial Statements requires management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. The estimates and associated assumptions are based on historical experience and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis of making the judgments about carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Management has applied significant estimates and judgments related to the following:
The determination of net realizable value requires significant judgment, including consideration of factors such as shrinkage, the aging of and future demand for inventory, expected future selling price the Company expects to realize by selling the inventory, and the contractual arrangements with customers. Reserves for excess and obsolete inventory are based upon quantities on hand, projected volumes from demand forecasts and net realizable value.
F-15
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
The future realization of these inventories may be affected by market-driven changes that may reduce future selling prices. A change to these assumptions could impact the Company’s inventory valuation and gross profit.
The impact of inventory reserves is reflected in cost of sales.
From time to time, the Company partakes in sales agreements with suppliers in which it also purchases inventory. As part of the five-step revenue model, the Company assesses whether instances of bulk sales made to suppliers of goods have commercial substance and should be recognized as revenue, or whether they should be assessed under ASC 845, Nonmonetary Transactions, which requires management judgment to determine if the transaction has commercial substance.
In calculating share-based compensation expense, key estimates are used such as, the rate of forfeiture of options granted, the expected life of the option, the volatility of the Company’s stock price, and the risk-free interest rate.
In calculating the fair value of warrants issued, the Company includes key estimates such as the volatility of the Company’s stock price and the risk-free interest rate. The Company uses judgment to select methods used and in performing the fair value calculations at the initial measurement at issuance, as well as for subsequent measurement on a recurring basis.
The extent to which deferred tax assets can be recognized is based on an assessment of the probability of the Company generating future taxable income against which the deferred tax assets can be utilized. In addition, significant judgment is required in classifying transactions and assessing probable outcomes of tax positions taken, and in assessing the impact of any legal or economic limits or uncertainties in various tax jurisdictions.
Provisions for taxes are made using the best estimate of the amount expected to be paid based on a qualitative assessment of all relevant factors. The Company reviews the adequacy of these provisions at the end of the reporting period. It is possible, however, that at some future date, an additional liability could result from audits by taxing authorities. If the final outcome of these tax-related matters is different from the amounts that were initially recorded, such differences will affect the tax provisions in the period in which such determination is made.
The Company uses an income-based approach as necessary to assess the fair values of indefinite lived intangible assets, asset groups and reporting units for goodwill testing purposes. Under the income approach, fair value is based on the present value of estimated cash flows. An impaired asset is written down to its estimated fair value based on the most recent information available.
Fair value is determined as the amount that would be obtained from the sale of the asset in an arm’s length transaction between knowledgeable and willing parties. Determining the value in use requires the Company to estimate expected future cash flows associated with the assets and a suitable discount rate in order to calculate present value. A number of factors, including historical results, business plans, forecasts, and market data are used to determine the fair value of the reporting unit and intangible assets.
The Company evaluates the recoverability of long-lived assets, including property and equipment, ROU assets, and definite lived intangible assets, whether events or changes in circumstances indicate that the carrying value of the asset, or asset group, may not be recoverable. Judgment is required to evaluate whether one or more indicators of potential impairment exist. The assets are assessed for impairment based on the estimated future undiscounted cash flows expected to result from the use of the asset and its eventual disposition. If the carrying value of an asset
F-16
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
exceeds its estimated future undiscounted cash flows, an impairment loss is recorded for the excess of the asset’s carrying value over its fair value.
Classification of an acquisition as a business combination or an asset acquisition depends on whether the asset acquired constitutes a business, which can be a complex judgment. The Company has determined that its acquisitions in Note 5 are business combinations under ASC, 805 Business Combinations.
In a business combination, substantially all identifiable assets, liabilities and contingent liabilities acquired are recorded at the date of acquisition at their respective fair values. One of the most significant areas of judgment and estimation relates to the determination of the fair value of these assets and liabilities, including the fair value of contingent consideration, if applicable. If any intangible assets are identified, depending on the type of intangible asset and the complexity of determining its fair value, the Company may utilize an independent external valuation expert to develop the fair value, using appropriate valuation techniques, which are generally based on a forecast of the total expected future net cash flows. These valuations are linked closely to the assumptions made by management regarding the future performance of the assets concerned and any changes in the discount rate applied.
Contingent consideration payable as the result of a business combination is recorded at the date of acquisition at fair value. The fair value of contingent consideration is subject to significant judgment and estimates, such as projected future revenue. Subsequent changes to the fair value of contingent consideration are measured at each reporting date, with changes recognized through profit or loss.
Lease payments are discounted using the rate implicit in the lease if that rate is readily available. If that rate cannot be easily determined, the lessee is required to use its incremental borrowing rate. The incremental borrowing rate is the rate of interest that the Company estimates it would have to pay on a collateralized basis to borrow an amount equal to the lease payments under similar terms. The Company calculates its incremental borrowing rate as the interest rate the Company would pay to borrow funds necessary to obtain an asset of similar value over similar terms taking into consideration the economic factors and the credit risk rating at the commencement date of the lease.
When determining the appropriate basis of accounting for the Company’s interests in affiliates, the Company makes judgments about the degree of influence that it exerts directly or through an arrangement over the investees’ relevant activities.
The Coronavirus Aid, Relief, and Economic Security Act ("CARES Act") provides for an Employee Retention Tax Credit ("ERC") which is a refundable tax credit for businesses that continued to pay employees while shut down due to the COVID-19 pandemic, or had significant declines in gross receipts from March 13, 2020 to December 31, 2021. Eligible employers were entitled to claim the ERC on an original or adjusted employment tax return for a period within those dates. The Company has elected to account for the ERC as a government grant. There is limited grant accounting guidance within U.S. GAAP that is applicable to for-profit entities. Therefore, the Company has elected to follow the grant accounting model in International Accounting Standard ("IAS") 20, Accounting for Government Grants and Disclosure of Government Assistance. Accordingly, the Company recognized government grants for which there is a reasonable assurance of compliance with grant conditions and receipt of credits and has therefore recognized a receivable for the total credit amount on the Consolidated Balance Sheet as of December 31, 2022 (refer to Note 4). The determination of the collectability of the ERC requires significant judgment, including assessment of the Company's eligibility based on the facts and circumstances. No formal determination has been made regarding the Company’s eligibility to receive ERC or if companies in the
F-17
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
cannabis industry are generally eligible to receive ERC. There is a risk that a determination could be made that the Company, due to its operations or the nature of its business, is not eligible for the ERC distributions it has received.
The Company consolidates legal entities in which it holds a controlling financial interest. Determining whether it has a controlling financial interest in VIE is subject to significant judgment and estimates. There is inherent uncertainty in evaluating who has the power to direct the activities of the VIE that most significantly impact the entity's economic performance. Our considerations include, but are not limited to, voting interests of the VIE, management, service and other agreements with the VIE, involvement in the VIE’s initial design and the existence of explicit or implicit financial guarantees. Management has applied significant judgment when evaluating the facts and circumstances of the VIE (Note 3).
Management determines the current expected credit losses by evaluating individual receivable balances along with the consideration of other financial and current economic conditions. Accounts receivable are written off when deemed uncollectible. Recoveries of accounts receivable previously written off are recorded as income when received. All receivables are expected to be collected within one year of the balance sheet date.
(aa) New standards, amendments and interpretations adopted
F-18
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
3. Consolidation
In connection with the listing of the Common Shares on the TSX, the Company reorganized its ownership structure to segregate the Company’s Canadian retail operations from TerrAscend's cultivation and manufacturing operations in the United States (the "Reorganization"). Following the completion of the Reorganization, the Company owns
In connection with the Reorganization, TerrAscend issued and sold, on a private placement basis, Class A shares in the capital of TerrAscend ("Class A Shares") for aggregate gross proceeds of $
The Issuer determined TerrAscend is a VIE, as all of the Company’s U.S. activities continue to be conducted on behalf of the Company which has disproportionately few voting rights. After conducting an analysis of the following VIE factors; purpose and design of the VIE, the Protection Agreement in place, the structure of the Company's board of directors (the "Board"), and substantive kick-out rights of the holders of the Class A Shares, it was determined that the Company has the power to direct the activities of TerrAscend. In addition, given the structure of the Class A Shares where all of the losses and substantially all of the benefits of TerrAscend are absorbed by the Company, the Company consolidates as the primary beneficiary in accordance with ASC 810, Consolidation.
The Company's U.S. operations are consolidated through the VIE model. Therefore, substantially all of the Company's current assets, non-current assets, current liabilities and non-current liabilities are consolidated through the VIE model. The Company's assets and liabilities that are not consolidated through the VIE model include convertible debt, derivative liability and assets and liabilities from discontinued operations. The Company also consolidates a minimal amount of assets and liabilities within Canada, see Note 21 for more information.
4. Accounts receivable, net
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Trade receivables |
|
$ |
|
|
$ |
|
||
Sales tax receivable |
|
|
|
|
|
|
||
Other receivables |
|
|
|
|
|
|
||
Provision for current expected credit losses |
|
|
( |
) |
|
|
( |
) |
Total receivables, net |
|
$ |
|
|
$ |
|
||
During the year ended December 31, 2022, the Company had an ERC for qualified wages of $
F-19
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
$
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Trade receivables |
|
$ |
|
|
$ |
|
||
Less: provision for current expected credit losses |
|
|
( |
) |
|
|
( |
) |
Total trade receivables, net |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Of which |
|
|
|
|
|
|
||
Current |
|
|
|
|
|
|
||
31-90 days |
|
|
|
|
|
|
||
Over 90 days |
|
|
|
|
|
|
||
Less: current expected credit losses |
|
|
( |
) |
|
|
( |
) |
Total trade receivables, net |
|
$ |
|
|
$ |
|
||
The over 90 days aged balance relates mainly to
The following is a roll-forward of the provision for sales returns and allowances related to trade accounts receivable:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Beginning of the year |
|
$ |
|
|
$ |
|
||
Provision for sales returns |
|
|
|
|
|
|
||
Expected credit losses |
|
|
|
|
|
|
||
Write-offs charged against provision |
|
|
( |
) |
|
|
( |
) |
Total provision for current expected credit losses |
|
$ |
|
|
|
|
||
5. Acquisitions
2023 Acquisitions
AMMD
On January 27, 2023, in order to start its retail footprint in Maryland, the Company acquired Allegany Medical Marijuana Dispensary ("AMMD"), a medical dispensary located in Cumberland, Maryland from Moose Curve Holdings, LLC (the "AMMD Acquisition"). Pursuant to the terms of the agreement, the Company acquired a
F-20
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
long-term lease with the option to purchase the real estate and repayments of indebtedness and transaction expenses on behalf of AMMD of $
The following table presents the fair value of assets acquired and liabilities assumed as of the January 27, 2023 acquisition date and allocation of the consideration to net assets acquired:
Cash and cash equivalents |
|
$ |
|
|
Inventory |
|
|
|
|
Prepaid expense |
|
|
|
|
Operating right of use asset |
|
|
|
|
Fixed assets |
|
|
|
|
Intangible asset |
|
|
|
|
Goodwill |
|
|
|
|
Accounts payable and accrued liabilities |
|
|
( |
) |
Deferred tax liability |
|
|
( |
) |
Corporate income taxes payable |
|
|
( |
) |
Operating lease liability |
|
|
( |
) |
Net assets acquired |
|
$ |
|
|
|
|
|
|
|
Cash |
|
|
|
|
Working capital adjustment |
|
|
( |
) |
Total consideration |
|
$ |
|
|
The acquired intangible assets include a medical license, which is treated as a definite-lived intangible asset and amortized over a
The consideration paid reflected the synergies, economies of scale, and workforce. These benefits were not recognized separately from goodwill because they do not meet the recognition criteria for identifiable intangible assets. None of the goodwill recognized is expected to be deductible for income tax purposes.
Costs related to this transaction were $
On a standalone basis, had the Company acquired the business on January 1, 2023, sales estimates would have been $
Peninsula
On June 28, 2023, in order to expand its retail footprint in Maryland, the Company closed the acquisition of Derby 1, LLC ("Peninsula"), a dispensary located in Salisbury, Maryland (the "Peninsula Acquisition"). Pursuant to the terms of the agreement, the Company acquired a
The Peninsula Share Consideration was subject to a statutory lock-up restriction of 6 months, and therefore, a share restriction discount was considered in determining the fair value of the Peninsula Share Consideration on the date of issuance, using the Finnerty Model.
Pursuant to the terms of the agreement, the Company agreed that if within
F-21
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
the remaining Common Shares is less than $
The following table presents the fair value of assets acquired and liabilities assumed as of the June 28, 2023 acquisition date and allocation of the consideration to net assets acquired:
|
|
|
|
|
Cash and cash equivalents |
|
|
|
|
Inventory |
|
|
|
|
Prepaid expense |
|
|
|
|
Operating right of use asset |
|
|
|
|
Fixed assets |
|
|
|
|
Intangible asset |
|
|
|
|
Goodwill |
|
|
|
|
Accounts payable and accrued liabilities |
|
|
( |
) |
Loans payable |
|
|
( |
) |
Operating lease liability |
|
|
( |
) |
Net assets acquired |
|
$ |
|
|
|
|
|
|
|
Cash |
|
|
|
|
Common shares of TerrAscend |
|
|
|
|
Loans payable |
|
|
|
|
Contingent consideration |
|
|
|
|
Total consideration |
|
$ |
|
|
The acquired intangible assets include a license, which is treated as a definite-lived intangible asset and amortized over a
The consideration paid reflected the synergies, economies of scale, and workforce. These benefits were not recognized separately from goodwill because they do not meet the recognition criteria for identifiable intangible assets. None of the goodwill recognized is expected to be deductible for income tax purposes.
Costs related to this transaction were $
On a standalone basis, had the Company acquired the business on January 1, 2023, sales estimates would have been $
Blue Ridge
On June 30, 2023, in order to expand its retail footprint in Maryland, the Company closed the acquisition of Hempaid, LLC ("Blue Ridge"), a medical dispensary located in Parkville, Maryland (the "Blue Ridge Acquisition"). The Company has plans to relocate Blue Ridge to a new, high-traffic retail center. Pursuant to the terms of the agreement, the Company acquired a
F-22
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
Cash Consideration"). The Blue Ridge Cash Consideration included repayments of indebtedness and transaction expenses on behalf of Blue Ridge of $
The following table presents the fair value of assets acquired and liabilities assumed as of the June 30, 2023 acquisition date and allocation of the consideration to net assets acquired:
|
|
|
|
|
Inventory |
|
|
|
|
Prepaid expense |
|
|
|
|
Operating right of use asset |
|
|
|
|
Intangible asset |
|
|
|
|
Goodwill |
|
|
|
|
Other asset |
|
|
|
|
Corporate income tax payable |
|
|
( |
) |
Deferred tax liability |
|
|
( |
) |
Accounts payable and accrued liabilities |
|
|
( |
) |
Operating lease liability |
|
|
( |
) |
Liability on uncertain tax position |
|
|
( |
) |
Net assets acquired |
|
$ |
|
|
|
|
|
|
|
Cash |
|
|
|
|
Loans payable |
|
|
|
|
Total consideration |
|
$ |
|
|
The acquired intangible assets include a license, which is treated as a definite-lived intangible asset and amortized over a
The consideration paid reflected the synergies, economies of scale, and workforce. These benefits were not recognized separately from goodwill because they do not meet the recognition criteria for identifiable intangible assets. None of the goodwill recognized is expected to be deductible for income tax purposes.
Costs related to this transaction were $
On a standalone basis, had the Company acquired the business on January 1, 2023, sales estimates would have been $
Herbiculture
On July 10, 2023, in order to expand its retail footprint in Maryland, the Company closed the acquisition of Herbiculture Inc. (“Herbiculture”), a medical dispensary in Maryland (the "Herbiculture Acquisition"). Pursuant to the terms of the agreement, the Company acquired
F-23
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
The following table presents the fair value of assets acquired and liabilities assumed as of the July 10, 2023 acquisition date and allocation of the consideration to net assets acquired:
Inventory |
|
$ |
|
|
Prepaid expense |
|
|
|
|
Accounts receivable |
|
|
|
|
Fixed assets |
|
|
|
|
Operating right of use asset |
|
|
|
|
Intangible asset |
|
|
|
|
Goodwill |
|
|
|
|
Deferred tax liability |
|
|
( |
) |
Accounts payable and accrued liabilities |
|
|
( |
) |
Corporate income taxes payable |
|
|
( |
) |
Operating lease liability |
|
|
( |
) |
Liability on uncertain tax position |
|
|
( |
) |
Net assets acquired |
|
$ |
|
|
|
|
|
|
|
Cash |
|
|
|
|
Loans payable |
|
|
|
|
Total consideration |
|
$ |
|
|
The acquired intangible assets include a license, which is treated as a definite-lived intangible asset and amortized over a
The consideration paid reflected the synergies, economies of scale, and workforce. These benefits were not recognized separately from goodwill because they do not meet the recognition criteria for identifiable intangible assets. None of the goodwill recognized is expected to be deductible for income tax purposes.
Costs related to this transaction were $
On a standalone basis, had the Company acquired the business on January 1, 2023, sales estimates would have been $
2022 Acquisitions
Pinnacle
On August 23, 2022, in order to expand its retail footprint in Michigan, the Company acquired all of the outstanding equity interests of KISA Enterprises MI, LLC ("Pinnacle") ("Pinnacle Acquisition"), a dispensary chain operator in Michigan, and related real estate assets from KISA Holdings, LLC, for total consideration of $
Pursuant to the terms of the agreement, Pinnacle Earn-Out Payment required the Company to make an
F-24
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
The following table presents the fair value of assets acquired and liabilities assumed as of the August 23, 2022 acquisition date and allocation of the consideration to net assets acquired:
|
|
$ |
|
|
Cash and cash equivalents |
|
|
|
|
Inventory |
|
|
|
|
Prepaid expenses and other current assets |
|
|
|
|
Property and equipment |
|
|
|
|
Operating right of use asset |
|
|
|
|
Intangible assets |
|
|
|
|
Goodwill |
|
|
|
|
Accounts payable and accrued liabilities |
|
|
( |
) |
Corporate income taxes payable |
|
|
( |
) |
Operating lease liability |
|
|
( |
) |
Deferred revenue |
|
|
( |
) |
Deferred tax liability |
|
|
( |
) |
Net assets acquired |
|
|
|
|
|
|
|
|
|
Consideration paid in cash |
|
|
|
|
Promissory note payable |
|
|
|
|
Contingent consideration payable |
|
|
|
|
Common shares of TerrAscend |
|
|
|
|
Working capital adjustment |
|
|
( |
) |
Total consideration |
|
|
|
|
The acquired intangible assets include retail licenses, which are treated as definite-lived intangible assets and amortized over a
The consideration paid reflected the synergies, economies of scale, and workforce. These benefits were not recognized separately from goodwill because they do not meet the recognition criteria for identifiable intangible assets. None of the goodwill recognized is expected to be deductible for income tax purposes.
Costs related to this transaction were $
Gage
On March 10, 2022, in order to expand its footprint in key markets, the Company closed the acquisition ("Gage Acquisition") of Gage Growth ("Gage"). Pursuant to the terms of an arrangement agreement between the Company and Gage ("Gage Arrangement Agreement"), the Company acquired all of the issued and outstanding subordinate voting shares of Gage. Pursuant to the terms of the Gage Arrangement Agreement, Gage shareholders received
F-25
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
subject to lock-up of $
The following table presents the fair value of assets acquired and liabilities assumed as of the March 10, 2022 acquisition date and allocation of the consideration to net assets acquired:
|
|
$ |
|
|
Cash and cash equivalents |
|
|
|
|
Restricted cash |
|
|
|
|
Accounts receivable |
|
|
|
|
Inventory |
|
|
|
|
Prepaid expenses and other assets |
|
|
|
|
Property and equipment |
|
|
|
|
Operating right of use asset |
|
|
|
|
Deposits |
|
|
|
|
Intangible assets |
|
|
|
|
Goodwill |
|
|
|
|
Investments |
|
|
|
|
Accounts payable and accrued liabilities |
|
|
( |
) |
Corporate income taxes payable |
|
|
( |
) |
Operating lease liability |
|
|
( |
) |
Finance lease liability |
|
|
( |
) |
Deferred revenue |
|
|
( |
) |
Loans payable |
|
|
( |
) |
Deferred tax liability |
|
|
( |
) |
Financing obligations |
|
|
( |
) |
Other liabilities |
|
|
( |
) |
Net assets acquired |
|
|
|
|
|
|
|
|
|
Common Shares of TerrAscend |
|
|
|
|
Fair value of other equity instruments |
|
|
|
|
Fair value of warrants classified as liabilities |
|
|
|
|
Total consideration |
|
|
|
|
The acquired intangible assets include cultivation and processing licenses, as well as retail licenses, which are treated as definite-lived intangible assets and are amortized over a
The consideration paid reflected the synergies, economies of scale, and workforce. These benefits were not recognized separately from goodwill because they do not meet the recognition criteria for identifiable intangible assets. None of the goodwill recognized is expected to be deductible for income tax purposes.
Costs related to this transaction were $
On a standalone basis, had the Company acquired the business on January 1, 2022, sales estimates would have been $
F-26
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
Contingent consideration
Contingent consideration recorded relates to the Company’s business acquisitions. Contingent consideration is based upon the potential earnout of the underlying business unit and is measured at fair value using a projection model for the business and the formulaic structure for determining the consideration under the terms of the agreement.
The balance of contingent consideration is as follows:
|
|
State Flower |
|
|
Apothecarium |
|
|
KCR |
|
|
Pinnacle |
|
|
Peninsula |
|
|
Total |
|
||||||
Carrying amount, December 31, 2021 |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
||||
Amount recognized on acquisition |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
Payments of contingent consideration |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Loss (gain) on revaluation of contingent consideration |
|
|
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
Carrying amount, December 31, 2022 |
|
$ |
|
|
$ |
|
|
|
— |
|
|
$ |
|
|
$ |
— |
|
|
$ |
|
||||
Amount recognized on acquisition |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|||
Payments of contingent consideration |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
Gain on revaluation of contingent consideration |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
Carrying amount, December 31, 2023 |
|
$ |
|
|
$ |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
|
|
$ |
|
||||
Less: current portion |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Non-current contingent consideration |
|
$ |
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
- |
|
|
$ |
- |
|
The contingent consideration for ABI SF, LLC ("State Flower") was calculated based on fiscal year 2021 revenue and the final earn-out was calculated as of December 31, 2021. During the year ended December 31, 2022, the Company made payments of $
During the year ended December 31, 2023, the Company issued
During the year ended December 31, 2022, the fair value of the contingent consideration related to the acquisition of Guadco, LLC and KCR Holdings LLC (collectively, "KCR") was reduced to $nil, as it was determined that it was more likely than not that the earnout criteria would not be met.
6. Inventory
The Company’s inventory of dry cannabis and oil includes both purchased and internally produced inventory. The Company’s inventory is comprised of the following items:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Raw materials |
|
$ |
|
|
$ |
|
||
Finished goods |
|
|
|
|
|
|
||
Work in process |
|
|
|
|
|
|
||
Accessories, supplies and consumables |
|
|
|
|
|
|
||
|
|
$ |
|
|
$ |
|
||
During the year ended December 31, 2023, the Company adjusted inventory by $
On February 4, 2022, more than
F-27
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
In addition, during the year ended December 31, 2022, the Company wrote down its inventory by $
7. Discontinued operations
The Company determined to make available for sale the asset groups related to TerrAscend Canada's Licensed Producer business. As a result, all prior year amounts from discontinued operations have been reclassified for consistency with the current year presentation.
As of December 31, 2023 and December 31, 2022, the major classes of assets and liabilities from discontinued operations included the following:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Land |
|
$ |
- |
|
|
$ |
|
|
Buildings & improvements |
|
|
|
|
|
|
||
Office furniture & equipment |
|
|
|
|
|
|
||
Total assets held for sale |
|
$ |
- |
|
|
$ |
|
|
|
|
|
|
|
|
|
||
Prepaid expenses and other current assets |
|
|
|
|
|
|
||
Current assets from discontinued operations |
|
$ |
- |
|
|
$ |
|
|
|
|
|
|
|
|
|
||
Accounts payable and accrued liabilities |
|
|
- |
|
|
|
|
|
Loans payable |
|
|
|
|
|
|
||
Current liabilities from discontinued operations |
|
$ |
- |
|
|
$ |
|
|
The results of operations for the discontinued operations includes revenues and expenses directly attributable to the operations disposed. Corporate and administrative expenses, including interest expense, not directly attributable to the operations were not allocated to TerrAscend Canada's Licensed Producer business.
|
|
For the Year Ended |
|
|||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
|
|
|
|
|
|
|
||
Revenue, net |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
||
Cost of Sales |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
||
Gross profit |
|
|
— |
|
|
|
( |
) |
|
|
|
|
|
|
|
||
Operating expenses: |
|
|
|
|
|
|
||
General and administrative |
|
|
|
|
|
|
||
Amortization and depreciation |
|
|
|
|
|
|
||
Impairment of property and equipment |
|
|
|
|
|
— |
|
|
Total operating expenses |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Loss from discontinued operations |
|
|
( |
) |
|
|
( |
) |
Other expense |
|
|
|
|
|
|
||
Finance and other expenses |
|
|
|
|
|
|
||
Net loss from discontinued operations |
|
$ |
( |
) |
|
$ |
( |
) |
Asset Specific Impairment
The Company evaluates the recoverability of property and equipment whenever events or changes in circumstances indicate that the carrying value of the asset or asset group may not be recoverable. See – Impairment of long-lived assets information within this note for detailed information on the Company’s impairment assessment of its property and equipment. The impairment losses discussed below were included in (loss) income from discontinued operations on the Company's Consolidated Statements of Operations and Comprehensive Income (Loss).
F-28
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
Certain assets of TerrAscend Canada were determined to be held for sale as of December 31, 2022 as they met the criteria under ASC 360, Property, Plant and Equipment. TerrAscend Canada operated out of a
Additionally, during the year ended December 31, 2022, the Company recorded impairment of $
8. Property and equipment, net
Property and equipment consisted of:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Land |
|
$ |
|
|
$ |
|
||
Assets in process |
|
|
|
|
|
|
||
Buildings & improvements |
|
|
|
|
|
|
||
Machinery & equipment |
|
|
|
|
|
|
||
Office furniture & equipment |
|
|
|
|
|
|
||
Assets under finance leases |
|
|
|
|
|
|
||
Total cost |
|
|
|
|
|
|
||
Less: accumulated depreciation |
|
|
( |
) |
|
|
( |
) |
Property and equipment, net |
|
$ |
|
|
$ |
|
||
Assets in process represent construction in progress related to both cultivation and dispensary facilities not yet completed, or otherwise not placed in service. During the year ended December 31, 2023, the Company determined that the carrying value of the certain assets in process may not be recoverable. As a result, the Company recorded an impairment loss of $
During the years ended December 31, 2023, and December 31, 2022, borrowing costs were
Depreciation expense was $
F-29
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
9. Intangible assets, net and goodwill
Intangible assets consisted of the following:
At December 31, 2023 |
|
Gross Carrying Amount |
|
|
Accumulated Amortization |
|
|
Net Carrying Amount |
|
|||
Finite lived intangible assets |
|
|
|
|
|
|
|
|
|
|||
Software |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||
Licenses |
|
|
|
|
|
( |
) |
|
|
|
||
Non-compete agreements |
|
|
|
|
|
( |
) |
|
|
— |
|
|
Total finite lived intangible assets |
|
|
|
|
|
( |
) |
|
|
|
||
Indefinite lived intangible assets |
|
|
|
|
|
|
|
|
|
|||
Brand intangibles |
|
|
|
|
|
— |
|
|
|
|
||
Total indefinite lived intangible assets |
|
|
|
|
|
— |
|
|
|
|
||
Intangible assets, net |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||
At December 31, 2022 |
|
Gross Carrying Amount |
|
|
Accumulated Amortization |
|
|
Net Carrying Amount |
|
|||
Finite lived intangible assets |
|
|
|
|
|
|
|
|
|
|||
Software |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||
Licenses |
|
|
|
|
|
( |
) |
|
|
|
||
Brand intangibles |
|
|
|
|
|
( |
) |
|
|
— |
|
|
Non-compete agreements |
|
|
|
|
|
( |
) |
|
|
|
||
Total finite lived intangible assets |
|
|
|
|
|
( |
) |
|
|
|
||
Indefinite lived intangible assets |
|
|
|
|
|
|
|
|
|
|||
Brand intangibles |
|
|
|
|
|
— |
|
|
|
|
||
Total indefinite lived intangible assets |
|
|
|
|
|
— |
|
|
|
|
||
Intangible assets, net |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||
Amortization expense was $
Estimated future amortization expense for finite lived intangible assets for the next five years is as follows:
2024 |
|
$ |
|
|
2025 |
|
|
|
|
2026 |
|
|
|
|
2027 |
|
|
|
|
2028 |
|
|
|
As of December 31, 2023, the weighted average amortization period remaining on intangible assets was
The Company's goodwill is allocated to one reportable segment.
Balance at December 31, 2021 |
|
$ |
|
|
Additions at acquisition date |
|
|
|
|
Impairment of goodwill |
|
|
( |
) |
Balance at December 31, 2022 |
|
$ |
|
|
Additions at acquisition date |
|
|
|
|
Impairment of goodwill |
|
|
( |
) |
Balance at December 31, 2023 |
|
$ |
|
F-30
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
Impairment of Intangible Assets
The Company recorded the following impairment losses by category of intangible assets:
|
|
|
|
|
|
|
|
|
|
|||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Finite lived intangible assets |
|
|
|
|
|
|
|
|
|
|||
Software |
|
$ |
— |
|
|
|
— |
|
|
$ |
|
|
Licenses |
|
|
|
|
|
|
|
|
— |
|
||
Customer Relationships |
|
|
— |
|
|
|
— |
|
|
|
|
|
Non-compete agreements |
|
|
— |
|
|
|
— |
|
|
|
|
|
Total impairment of finite lived intangible assets |
|
|
|
|
|
|
|
|
|
|||
Indefinite lived intangible assets |
|
|
|
|
|
|
|
|
|
|||
Brand intangibles |
|
|
|
|
|
|
|
|
|
|||
Total impairment of indefinite lived intangible assets |
|
|
|
|
|
|
|
|
|
|||
Total impairment of intangible assets |
|
$ |
|
|
$ |
|
|
$ |
|
|||
The Company evaluates the recoverability of long-lived assets, including definite lived intangible assets, whether events or changes in circumstances indicate that the carrying value of the asset may not be recoverable.
During the year ended December 31, 2023, the Company determined that changes in market expectations of cash flows in its Michigan and California businesses, as well as increased competition and supply in those states, were indicators that an impairment test was appropriate for each of these reporting units.
During the year ended December 31, 2022, the Company determined that changes in market expectations of cash flows in its Michigan, Pennsylvania and California businesses, as well as increased competition and supply in those states, were indicators that an impairment test was appropriate for each of these reporting units.
Impairment of indefinite lived assets
Indefinite lived intangible assets are reviewed for impairment annually and whether there are events or changes in circumstances that indicate that the carrying amount has been impaired.
The impairment indicators previously noted for Michigan indicate that the fair value of the intangible assets associated with the Gage brand are more likely than not lower than the carrying value. As such, the Company performed an impairment analysis and determined the fair value of its brand intangible assets using the relief of royalty method on the following key assumptions:
As a result of the quantitative analysis, the Company recognized impairment of the Gage brand of $
Additionally, for the year ended December 31, 2023, the Company noted that fair value of the intangible assets associated with Gage banner is also impacted by the impairment indicators previously noted. As such, the Company performed an impairment analysis using a discounted cash flow model and determined the fair value is more likely than not lower than the carrying value. As a result of the quantitative analysis, the Company recognized impairment of $
F-31
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
During the year ended December 31, 2021, the Company made the decision to undertake a strategic review process to explore, review, and evaluate potential alternatives for Arise Bioscience, Inc. (“Arise”). The Company also determined that the estimated future cash flows for the business did not support the carrying value of the intangible assets, and therefore recorded impairment of intangible assets of $
Impairment of finite lived intangible assets
When testing for the impairment of finite lived intangible assets, management first calculates the undiscounted cash flows relating to each asset group. If the calculated undiscounted cash flow is less than the carrying value of the asset group, then impairment is indicated.
The fair value of each asset group was determined using cash flows expected to be generated by market participants, discounted at weighted average cost of capital. The fair value of the specific assets that were impaired was determined using the multi period excess earnings method or a discounted cash flow approach based on the following key assumptions:
During the year ended December 31, 2023, for the California asset group, the Company compared the carrying value of the retail license to its fair value and determined that the carrying value exceeded the fair value. The Company recorded impairment charges of $
During the year ended December 31, 2022, for the Michigan reporting unit, the Company determined the fair value of the asset groups and allocates the impairment to the assets, being the (i) cultivation and processing licenses, and (ii) retail licenses, acquired through the Gage Acquisition. The Company compared the carrying value of the assets to its fair value and determined that the carrying value exceeded the fair value for both the retail and the cultivation and processing licenses. As such, the Company recorded impairment charges of $
Impairment of Goodwill
Goodwill is reviewed for impairment annually and whenever there are events or changes in circumstances that indicate the carrying value has been impaired.
Based on the indicators of impairment noted previously, the Company determines whether a quantitative impairment test is necessary to assess that the fair value of its reporting units are more likely than not lower than its carrying value at some of its reporting units.
The following significant assumptions were applied in the determination of the fair value of the reporting units using a discounted cash flow model:
F-32
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
Based on the impairment indicators noted previously, during the year ended December 31, 2023, the Company recorded impairment of goodwill of $
During the year ended December 31, 2022, the Company recorded impairment of goodwill of $
As a result of the Company’s decision to undertake a strategic review of its Florida business, the Company recorded impairment of goodwill of $
10. Loans payable
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Chicago Atlantic term loan due |
|
|
|
|
|
|
||
Principal amount |
|
$ |
|
|
$ |
|
||
Deferred financing cost |
|
|
- |
|
|
|
- |
|
Net carrying amount |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Ilera term loan due |
|
|
|
|
|
|
||
Principal amount |
|
$ |
|
|
$ |
|
||
Deferred financing cost |
|
|
( |
) |
|
|
( |
) |
Net carrying amount |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Stearns loan due |
|
|
|
|
|
|
||
Principal amount |
|
$ |
|
|
$ |
- |
|
|
Deferred financing cost |
|
|
( |
) |
|
|
- |
|
Net carrying amount |
|
$ |
|
|
$ |
- |
|
|
|
|
|
|
|
|
|
||
Pelorus term loan due |
|
|
|
|
|
|
||
Principal amount |
|
$ |
|
|
$ |
|
||
Deferred financing cost |
|
|
( |
) |
|
|
( |
) |
Net carrying amount |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Maryland Acquisition loans (1) |
|
|
|
|
|
|
||
Principal amount |
|
$ |
|
|
$ |
- |
|
|
Unamortized discount |
|
|
( |
) |
|
|
- |
|
Net carrying amount |
|
$ |
|
|
$ |
- |
|
|
|
|
|
|
|
|
|
||
Other loans |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Short-term debt |
|
$ |
|
|
$ |
|
||
Current portion of long-term debt |
|
$ |
|
|
|
|
||
Loans payable, current |
|
$ |
|
|
|
|
||
|
|
|
|
|
|
|
||
Loans payable, non-current |
|
$ |
|
|
$ |
|
||
Total loans payable |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
(1) For maturity breakout, refer to Maryland Acquisition Loans section below. |
|
|
|
|
|
|
||
Total interest paid on all loan payables was $
F-33
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
Chicago Atlantic Term Loan
In connection with the Gage Acquisition, the Company assumed a senior secured term loan (the "Chicago Atlantic Term Loan") with an acquisition date fair value of $
On August 10, 2022, the Chicago Atlantic Term Loan was amended as a result of the corporate restructure in conjunction with the Gage Acquisition. The amendment to the Chicago Atlantic Term Loan includes the addition of a borrower and guarantor under the term loan and a right of first offer in favor of the administrative agent for a refinancing of the term loan. This amendment was not considered extinguishment of debt under ASC 470, Debt. As a result of the amendment, the Company paid a loan amendment fee of $
On November 29, 2022, the Company repaid $
On June 9, 2023, the Company agreed to an amendment among other things, to (i) permit changes necessary for the TSX Transaction (as defined therein) and (ii) to permit certain indebtedness and waive certain tax provisions. This amendment was not considered extinguishment of debt under ASC 470, Debt.
Ilera Term Loan
On December 18, 2020, WDB Holding PA, a subsidiary of the TerrAscend, entered into a senior secured term loan with a syndicate of lenders in the amount of $
On April 28, 2022, the Ilera Term Loan was amended to provide WDB Holding PA with greater flexibility by resetting the minimum consolidated interest coverage ratio levels that must be satisfied at the end of each measurement period and extending the date in which WDB Holding PA is required to deliver its budget for the fiscal year ending December 31, 2021. In addition, the no-call period was extended from
On November 11, 2022, WDB Holding PA, the Company, TerrAscend USA and the subsidiary guarantors party to the Ilera Term Loan and the PA Agent (on behalf of the required lenders) entered into an amendment to the Ilera Term Loan, pursuant to which the parties agreed that WDB Holding PA's obligation to maintain the consolidated interest coverage ratio as set forth
F-34
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
in the Ilera Term Loan for the period ended September 30, 2022, shall not apply, subject to certain conditions, including (but not limited to) an obligation to enter into a subsequent amendment agreement on or before December 15, 2022, documenting certain enhancements and amendments to the Ilera Term Loan. In addition, WDB Holding PA offered a prepayment of $
On December 21, 2022, WDB Holding PA completed an amendment to reduce the Company's principal debt by $
On March 15, 2023, WDB Holding PA, in exchange for a fee in the amount of
On April 14, 2023, WDB Holding PA agreed to an amendment to the Ilera Term Loan to, among other things, to (i) permit changes necessary for the TSX Transaction (as defined therein), and (ii) to waive certain tax provisions.
On June 8, 2023, June 15, 2023, and June 29, 2023, WBD Holding PA made repayments of principal in the amounts of $
On June 22, 2023, WDB Holding PA further agreed to an amendment among other things, to (i) extend the next test date for the interest coverage ratio from June 30, 2023 to September 30, 2023, and (ii) amend the terms for which WDB Holding PA may incur certain indebtedness and liens.
On October 2, 2023, the Company made a mandatory prepayment of the Ilera Term Loan of $
On December 4, 2023, the parties entered into an amendment that requires WDB Holding PA to make a prepayment of $
In accordance with ASC 470, Debt, the amendments above were not considered extinguishment of debt.
Stearns Loan
On June 26, 2023, the Company closed on a $
Pelorus Term Loan
On October 11, 2022, subsidiaries of, TerrAscend, among others, entered into a loan agreement with Pelorus Fund REIT, LLC ("Pelorus") for a single-draw senior secured term loan (the “Pelorus Term Loan") in an aggregate principal amount of $
F-35
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
borrowers under the Pelorus Term Loan are guaranteed by the Company, TerrAscend USA and certain other subsidiaries of TerrAscend and are secured by all of the assets of TerrAscend's Maryland and New Jersey businesses, including certain real estate in Maryland and New Jersey, but excludes all MD dispensaries. The Pelorus Term Loan matures on October 11, 2027.
On April 17, 2023, TerrAscend NJ, LLC agreed to an amendment among other things, to (i) permit changes necessary for the TSX Transaction (as defined therein), and (ii) to waive certain tax provisions. On June 22, 2023, TerrAscend NJ, LLC further agreed to an amendment to permit certain indebtedness.
In accordance with ASC 470, Debt, the amendments above were not considered extinguishment of debt.
Maryland Acquisition Loans
On June 28, 2023, in connection with the Peninsula Acquisition, the Company assumed existing indebtedness in the form of a promissory note in the amount of $
On June 30, 2023, in connection with the Blue Ridge Acquisition, the Company entered into a promissory note in the amount of $
On July 10, 2023, in connection with the Herbiculture Acquisition, the Company entered into a promissory note in the amount of $
Other loans
Stadium Ventures
In connection with the Gage Acquisition, the Company assumed existing indebtedness in the form of a promissory note in the amount of $
Class A Shares of TerrAscend Growth
In connection with the Reorganization (see Note 3), TerrAscend issued $
F-36
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
Repurchase/Put Price, payable in cash or shares. The instrument is considered as a debt for accounting purposes due to the economic characteristics and risks.
Short-Term Debt
Pinnacle Loans
In connection with the Pinnacle Acquisition, the Company entered into
IHC Real Estate LP Loan
On June 26, 2023, the Company acquired the minority interest in IHC Real Estate LP and in connection therewith, the Company entered into a promissory note in the amount of $
Bay City Promissory Note
On October 30, 2023, the Company entered into a promissory note in the amount of $
Maturities of loans payable
Stated maturities of loans payable over the next five years are as follows:
|
|
December 31, 2023 |
|
|
2024 |
|
$ |
|
|
2025 |
|
|
|
|
2026 |
|
|
|
|
2027 |
|
|
|
|
2028 |
|
|
|
|
Thereafter |
|
|
- |
|
Total principal payments |
|
$ |
|
|
F-37
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
11. Leases
Amounts recognized in the Consolidated Balance Sheets were as follows:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Operating leases: |
|
|
|
|
|
|
||
Operating lease right-of-use assets |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Operating lease liability classified as current |
|
|
|
|
|
|
||
Operating lease liability classified as non-current |
|
|
|
|
|
|
||
Total operating lease liabilities |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Finance leases: |
|
|
|
|
|
|
||
|
$ |
|
|
$ |
|
|||
|
|
|
|
|
|
|
||
Lease obligations under finance leases classified as current |
|
|
|
|
|
|
||
Lease obligations under finance leases classified as non-current |
|
|
|
|
|
|
||
Total finance lease obligations |
|
$ |
|
|
$ |
|
||
The Company recognized operating lease expense of $
Other information related to operating leases consisted of the following:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Weighted-average remaining lease term (years) |
|
|
|
|
|
|
||
Operating leases |
|
|
|
|
|
|
||
Finance leases |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Weighted-average discount rate |
|
|
|
|
|
|
||
Operating leases |
|
|
% |
|
|
% |
||
Finance leases |
|
|
% |
|
|
% |
||
Supplemental cash flow information related to leases were as follows:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Cash paid for amounts included in measurement of operating lease liabilities |
|
$ |
|
|
$ |
|
||
Right-of-use assets obtained in exchange for operating lease obligations |
|
|
|
|
|
|
||
Cash paid for amounts included in measurement of finance lease liabilities |
|
|
|
|
|
|
||
Assets under finance leases obtained in exchange for finance lease obligations |
|
|
— |
|
|
|
|
|
F-38
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
Undiscounted lease obligations as of December 31, 2023 are as follows:
|
|
Operating |
|
|
Finance |
|
|
Total |
|
|||
2024 |
|
|
|
|
|
|
|
$ |
|
|||
2025 |
|
|
|
|
|
|
|
|
|
|||
2026 |
|
|
|
|
|
|
|
|
|
|||
2027 |
|
|
|
|
|
|
|
|
|
|||
2028 |
|
|
|
|
|
|
|
|
|
|||
Thereafter |
|
|
|
|
|
|
|
|
|
|||
Total lease payments |
|
|
|
|
|
|
|
|
|
|||
Less: interest |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Total lease liabilities |
|
$ |
|
|
$ |
|
|
$ |
|
|||
A sale-leaseback transaction occurs when an entity sells an asset it owns and then immediately leases the asset back from the buyer. The seller then becomes the lessee and the buyer becomes the lessor. Under ASC 842, Leases, both parties must assess whether the buyer-lessor has obtained control of the asset and a sale has occurred. Through the Gage Acquisition, the Company entered into leaseback transactions on
During the third fiscal quarter of 2023, five out of the six agreements were amended to remove the purchase option which qualified the transactions as a sale. As a result, the Company derecognized underlying assets of $
During the fourth quarter of 2023, the Company issued a promissory note to buy back the remaining property for $
12. Convertible debt
In June and August 2023, the Company closed the private placements of a total of
In accordance with ASC 815, Derivatives and Hedging, the conversion option was bifurcated from the host instrument as the instrument's strike price is denominated in a currency other than the functional currency of the Issuer. It was recorded at fair value, using the Black-Scholes Model (Note 23). The proceeds are allocated first to the conversion option based on its fair value of $
The following table summarizes the convertible debt activity for the year-ended December 31, 2023:
F-39
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
Balance at December 31, 2022 |
|
$ |
- |
|
Convertible debt proceeds, net of transaction costs |
|
|
|
|
Allocation to conversion option |
|
|
( |
) |
Interest and accretion |
|
|
|
|
Ending carrying amount at December 31, 2023 |
|
$ |
|
13. Shareholders’ equity
The Company is authorized to issue an unlimited number of Common Shares, an unlimited number of Proportionate Voting Shares, an unlimited number of Exchangeable Shares, and an unlimited number of Preferred Shares, issuable in series. The Board has the discretion to determine the rights, preferences, privileges, and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges, and liquidation preferences, of each class of the Company’s capital stock.
Unlimited Number of Preferred Shares
The Board has authorized the Company to issue an unlimited number of Series A, Series B, Series C and Series D Preferred Shares. The Preferred Shares of each series will, with respect to the payment of dividends and the distribution of assets in the event of the liquidation, dissolution or winding-up of the Company, whether voluntarily or involuntarily, rank on parity with the Preferred Shares of every other series, and will be entitled to preference over the Proportionate Voting Shares, Common Shares and Exchangeable Shares.
Voting Rights
Holders of Preferred Shares are not entitled to receive notice of, or to attend or to vote at any meeting of the shareholders of the Company.
Dividends
Holders of Preferred Shares are not entitled to receive any dividends, except that if the Company issues a dividend when necessary to comply with contractual provisions in respect of an adjustment to the conversion ratio in connection with any dividend paid on the Common Shares.
Conversion Rights
Holders of Preferred Shares are entitled to convert each outstanding Preferred Share into
The Preferred Shares will be automatically converted into Proportionate Voting Shares at the then-effective conversion ratio, instead of being redeemed for cash and other assets, in the event of a change in control.
Redemption Rights
The Company classified the Preferred Shares as permanent equity in the financial statements given that the terms do not obligate the Company to buy back the shares of Preferred Shares in exchange for cash or other assets, nor do the shares represent an obligation that must or may be settled with a variable number of shares, which are debt-like features. No other redemption provisions exist within the terms of the instrument.
Liquidation Preference
In the event of liquidation, dissolution, or winding up of the Company, whether voluntary or involuntary, or upon any other return of capital or distribution of the assets of the Company among its shareholders, in each case for the purposes of winding up its affairs, each Preferred Share entitles the holder thereof to receive and to be paid out of the assets of the Company available for distribution, before any distribution or payment may be made to a holder of any Common Shares, Proportionate Voting Shares, Exchangeable Shares or any other shares ranking junior in such liquidation, dissolution, or winding up to the Preferred
F-40
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
Shares, an amount per Preferred Share equal to the fair market value of the consideration paid for such Preferred Share upon issuance.
The Company’s Series A, Series B, Series C and Series D Preferred Shares have a liquidation preference that is initially equal to $
After payment to the holders of the Preferred Shares of the full liquidation preference to which they are entitled in respect of outstanding Preferred Shares (which, for greater certainty), have not been converted prior to such payment), such Preferred Shares will have no further right or claim to any of the assets of the Company.
The liquidation preference will be payable to holders of Preferred Shares in cash; provided, however, that to the extent the Company has, having exercised commercially reasonable efforts to make such payment, insufficient cash available to pay the liquidation preference in full in cash, the portion of the Liquidation Preference with respect to which the Company has insufficient cash may be paid in property or other assets of the Company. The value of any property or assets not consisting of cash that is distributed by the Company in satisfaction of any portion of the liquidation preference will equal the fair market value on the date of distribution. As of December 31, 2023, the Convertible Preferred Series A and B Shares had an aggregate liquidation value of $
Unlimited Number of Proportionate Voting Shares
Holders of Proportionate Voting Shares are entitled to receive, as and when declared by the Board, dividends in cash or property of the Company.
In the event of the liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary, or in the event of any other distribution of assets of the Company among its shareholders for the purpose of winding up its affairs, the holders of Proportionate Voting Shares are entitled to participate pari passu with the holders of Common Shares in an amount equal to the amount of such distribution per Common Share multiplied by 1,000.
Holders of Proportionate Voting Shares are not entitled to a right of first refusal to subscribe for, purchase or receive any part of any issue of shares, or bonds, debentures or other securities of the Company.
There may be no subdivision or consolidation of the Proportionate Voting Shares unless, simultaneously, the Common Shares and Exchangeable Shares are subdivided or consolidated using the same divisor or multiplier.
Unlimited Number of Exchangeable Shares
Voting Rights
Holders of Exchangeable Shares will not be entitled to receive notice of, attend or vote at meetings of the shareholders of the Company; provided that the holders of Exchangeable Shares will, however, be entitled to receive notice of meetings of shareholders called for the purpose of authorizing the dissolution of the Company or the sale of its assets, or a substantial part thereof, but holders of Exchangeable Shares will not be entitled to vote at such meeting of the shareholders of the Company.
Dividends
Holders of Exchangeable Shares will not be entitled to receive any dividends.
Dissolution
F-41
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
In the event of liquidation, dissolution or winding up of the Company, whether voluntary or involuntary, or any other distribution of assets of the Company among its shareholders for the purpose of winding-up its affairs, holders of Exchangeable Shares will not be entitled to receive any amount, property or assets of the Company.
Exchange Rights
Each issued and outstanding Exchangeable Share may at any time following the exchange start date applicable to the holder of such Exchangeable Share, at the option of the holder and subject to any restrictions or limitations, be exchanged for one Common Share.
Unlimited Number of Common Shares
Voting Rights
Dividend Rights
Holders of Common Shares are entitled to receive, subject to the rights of the holder of any other class of shares, any dividends declared by the Company. If, as and when dividends are declared by the directors, each Common Share will be entitled to 0.001 times the amount paid or distributed per Proportionate Voting Share (or, if a stock dividend is declared, each Common Share will be entitled to receive the same number of Common Shares per Common Share of Proportionate Voting Shares entitled to be received per Proportionate Voting Share).
Dissolution
In the event of liquidation, dissolution or winding up of the Company, whether voluntary or involuntary, or any other distribution of assets of the Company among its shareholders for the purpose of winding-up its affairs, the holders of the Common Shares will, subject to the rights of any other class of shares, be entitled to receive the remaining property of the Company on the basis that each Common Share will be entitled to 0.001 times the amount distributed per Proportionate Voting Share, but otherwise there is no preference or distinction among or between the Proportionate Voting Shares and the Common Shares.
Conversion Rights
Each issued and outstanding Common Share may at any time, at the option of the holder, be converted into
Description of Transactions:
Common Shares (Private Placements)
In June 2023, concurrently with convertible debenture placements (Note 12), the Company closed three tranches of private placements and issued
Detachable warrants issued in a bundled transaction are accounted for separately. Under ASC 815, Derivatives and Hedging, the detachable warrants meet the definition of derivative because the exercise price is denominated in a currency that is different from the functional currency of the Company. It was recorded at a fair value of $
F-42
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
On January 28, 2021, the Company completed a private placement and issued
Warrants
The following is a summary of the outstanding warrants for Common Shares:
|
|
Number of Common Share Warrants Outstanding |
|
|
Number of Common Share Warrants Exercisable |
|
|
Weighted Average Exercise Price $ |
|
|
Weighted Average Remaining Life (years) |
|
||||
Outstanding, December 31, 2020 |
|
|
|
|
|
|
|
$ |
|
|
|
|
||||
Exercised |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||
Outstanding, December 31, 2021 |
|
|
|
|
|
|
|
$ |
|
|
|
|
||||
Replacement warrants granted on acquisition of Gage |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Exercised |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||
Expired |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||
Outstanding, December 31, 2022 |
|
|
|
|
|
|
|
$ |
|
|
|
|
||||
Granted |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Expired |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
— |
|
|
Outstanding, December 31, 2023 |
|
|
|
|
|
|
|
$ |
|
|
|
|
||||
On December 9, 2022, the Company entered into an arrangement with Canopy USA, LLC ("Canopy USA") and certain of its subsidiaries to convert CAD$
The fair value of the New Warrants was determined using the Black-Scholes Model using the following inputs and assumptions:
|
|
December 9, 2022 |
|
|
Volatility |
|
|
% |
|
Risk-free interest rate |
|
|
% |
|
Expected life (years) |
|
|
||
Dividend yield |
|
|
% |
|
Pursuant to the terms of the Gage Acquisition, each holder of a warrant to purchase subordinate voting shares of Gage (a "Gage Warrant") was replaced with a warrant to purchase Common Shares (a "Replacement Warrant") on the basis of
F-43
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
The following is a summary of the outstanding warrant liabilities
|
|
Number of Common Share Warrants Outstanding |
|
|
Number of Common Share Warrants Exercisable |
|
|
Weighted Average Exercise Price $ |
|
|
Weighted Average Remaining Life (years) |
|
||||
Outstanding, December 31, 2021 |
|
|
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
Granted |
|
|
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
Outstanding, December 31, 2022 |
|
|
|
|
|
|
|
$ |
|
|
|
|
||||
Granted |
|
|
|
|
|
— |
|
|
|
|
|
|
|
|||
Expired |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
||
Outstanding, December 31, 2023 |
|
|
|
|
|
— |
|
|
$ |
|
|
|
|
|||
The following is a summary of the outstanding warrants for Proportionate Voting Shares. These warrants are exercisable for
|
|
Number of Proportionate Share Warrants Outstanding |
|
|
Number of Proportionate Share Warrants Exercisable |
|
|
Weighted Average Exercise Price $ |
|
|
Weighted Average Remaining Life (years) |
|
||||
Outstanding, December 31, 2020 |
|
|
|
|
|
|
|
$ |
|
|
|
|
||||
Granted |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|||
Exercised |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Outstanding, December 31, 2021 |
|
|
|
|
|
|
|
$ |
|
|
|
|
||||
Expired |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||
Outstanding, December 31, 2022 |
|
|
— |
|
|
|
— |
|
|
N/A |
|
|
N/A |
|
||
The expiration of the warrants for Proportionate Voting Shares resulted in an increase to additional paid in capital and a decrease to the accumulated deficit in the Consolidated Balance Sheets.
The following is a summary of the outstanding warrants for Preferred Shares. Each warrant is exercisable into
|
|
Number of Preferred Share Warrants Outstanding |
|
|
Number of Preferred Share Warrants Exercisable |
|
|
Weighted Average Exercise Price $ |
|
|
Weighted Average Remaining Life (years) |
|
||||
Outstanding, December 31, 2020 |
|
|
|
|
|
|
|
$ |
|
|
|
|
||||
Granted |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|||
Exercised |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||
Outstanding, December 31, 2021 |
|
|
|
|
|
|
|
$ |
|
|
|
|
||||
Exercised |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||
Outstanding, December 31, 2022 |
|
|
|
|
|
|
|
$ |
|
|
|
|
||||
Expired |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
— |
|
|
Outstanding, December 31, 2023 |
|
|
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
14. Share-based compensation plans
Share-based payments expense
Total share-based payments expense was as follows:
F-44
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
|
|
|
|
|
|
|
|
|
|
|||
|
For the Twelve Months Ended |
|
||||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Stock options |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Restricted share units |
|
|
|
|
|
|
|
$ |
|
|||
Total share-based payments |
|
$ |
|
|
$ |
|
|
$ |
|
|||
As of December 31, 2023, the total unrecognized compensation cost related to nonvested stock options was $
Stock Options
The Company’s
The options to purchase Common Shares outstanding noted below consist of service-based options granted to employees, the majority of which vest over a to
F-45
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
The following table summarizes the stock option activity:
|
|
Number of Stock Options |
|
|
Weighted average remaining contractual life (in years) |
|
|
Weighted Average Exercise Price (per share) $ |
|
Aggregate intrinsic value |
|
||||
Outstanding, December 31, 2020 |
|
|
|
|
|
|
|
$ |
|
$ |
|
||||
Granted |
|
|
|
|
|
|
|
|
|
|
|
||||
Exercised |
|
|
( |
) |
|
|
|
|
|
|
|
|
|||
Forfeited (1) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|||
Expired |
|
|
( |
) |
|
|
|
|
|
|
|
|
|||
Outstanding, December 31, 2021 |
|
|
|
|
|
|
|
$ |
|
$ |
|
||||
Granted |
|
|
|
|
|
|
|
|
|
|
|
||||
Replacement options granted on acquisition of Gage |
|
|
|
|
|
|
|
|
|
|
|
||||
Exercised |
|
|
( |
) |
|
|
|
|
|
|
|
|
|||
Forfeited (1) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|||
Expired |
|
|
( |
) |
|
|
|
|
|
|
|
|
|||
Outstanding, December 31, 2022 |
|
|
|
|
|
|
|
$ |
|
$ |
|
||||
Granted |
|
|
|
|
|
— |
|
|
|
|
|
— |
|
||
Exercised |
|
|
( |
) |
|
|
— |
|
|
|
|
|
— |
|
|
Forfeited |
|
|
( |
) |
|
|
— |
|
|
|
|
|
— |
|
|
Expired |
|
|
( |
) |
|
|
— |
|
|
|
|
|
— |
|
|
Outstanding, December 31, 2023 |
|
|
|
|
|
|
|
$ |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
||||
Exercisable, December 31, 2023 |
|
|
|
|
|
|
|
$ |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
||||
Nonvested, December 31, 2023 |
|
|
|
|
|
|
|
$ |
|
$ |
|
||||
(1)
The aggregate intrinsic value in the table above represents the total pre-tax intrinsic value (the difference between Company’s closing stock price at the end of the period and the exercise price, multiplied by the number of the in-the-money options) that would have been received by the option holders had all option holders exercised their in-the-money options on December 31, 2023, 2022, and 2021, respectively.
The total pre-tax intrinsic value (the difference between the market price of the Common Shares on the exercise date and the price paid by the options to exercise the option) related to stock options exercised is presented below:
|
For the Twelve Months Ended |
|
||||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Exercised |
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
|
March 10, 2022 |
|
|
Volatility |
|
|
||
Risk-free interest rate |
|
|
||
Expected life (years) |
|
|
||
Dividend yield |
|
|
% |
|
F-46
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Volatility |
|
|
|
|
|
|
||||||
Risk-free interest rate |
|
|
|
|
|
|
||||||
Expected life (years) |
|
|
|
|
|
|
||||||
Dividend yield |
|
|
% |
|
|
% |
|
|
% |
|||
Forfeiture rate |
|
|
% |
|
|
% |
|
|
||||
Volatility was estimated by using the historical volatility of the Company. The expected life in years represents the period of time that the options issued are expected to be outstanding. The risk-free rate is based on U.S. treasury bond issues with a remaining term approximately equal to the expected life of the options. Dividend yield is based on the fact that the Company has never paid cash dividends and does not expect to pay cash dividends in the foreseeable future.
The total estimated fair value of stock options that vested during the years ended December 31, 2023, 2022, and 2021, was $
Restricted Share Units
The Company’s RSU Plan was approved by the Board effective November 19, 2019. The RSU Plan governs the grant, administration and release of share units ("RSUs") which may be granted to Eligible Persons of the Company. Pursuant to the RSU Plan, the number of Common Shares that may be reserved for issuance under the RSU Plan and under any other share compensation plans of the Company, including the Stock Option Plan, may not exceed (in the aggregate)
The following table summarizes the activities for the RSUs:
|
|
Number of RSUs |
|
|
Number of RSUs vested |
|
|
Weighted average remaining contractual life (in years) |
|
|||
Outstanding, December 31, 2020 |
|
|
|
|
|
|
|
N/A |
|
|||
Granted |
|
|
|
|
|
— |
|
|
|
|
||
Vested |
|
|
( |
) |
|
|
— |
|
|
|
|
|
Forfeited |
|
|
( |
) |
|
|
— |
|
|
|
|
|
Outstanding, December 31, 2021 |
|
|
|
|
|
|
|
N/A |
|
|||
Granted |
|
|
|
|
|
— |
|
|
|
|
||
Vested |
|
|
( |
) |
|
|
— |
|
|
|
|
|
Forfeited |
|
|
( |
) |
|
|
— |
|
|
|
|
|
Outstanding, December 31, 2022 |
|
|
|
|
|
|
|
N/A |
|
|||
Granted |
|
|
|
|
|
— |
|
|
|
— |
|
|
Vested |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
Forfeited |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
Outstanding, December 31, 2023 |
|
|
|
|
|
— |
|
|
N/A |
|
||
Of the RSUs granted during the year ended December 31, 2023,
As of December 31, 2023, there was $
F-47
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
15. Non-controlling interest
Non-controlling interest consists mainly of TerrAscend's minority ownership interest in TerrAscend's New Jersey operations.
On June 26, 2023, the Company reduced its non-controlling interest through a buyout of the minority interest in IHC Real Estate LP (Note 10).
The transaction was accounted for as an equity transaction. The carrying amount of the non-controlling interest was adjusted by $
The following table summarizes the non-controlling interest activity for the years ended:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Opening carrying amount |
|
$ |
|
|
$ |
|
||
Capital distributions |
|
|
( |
) |
|
|
( |
) |
Acquisition of non-controlling interest |
|
|
( |
) |
|
|
— |
|
Net income attributable to non-controlling interest |
|
|
|
|
|
|
||
Ending carrying amount |
|
$ |
( |
) |
|
$ |
|
|
16. Related parties
The amounts due to/from related parties consisted of:
F-48
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
17. Income taxes
The domestic and foreign components of (loss) income from continuing operations before provision for income taxes are as follows:
|
For the years ended |
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Domestic |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
Foreign |
|
|
( |
) |
|
|
|
|
|
|
||
Income (loss) before income taxes |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|
The provision for income taxes consists of:
|
For the years ended |
|
||||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Current: |
|
|
|
|
|
|
|
|
|
|||
Federal |
|
|
|
|
|
|
|
|
|
|||
State |
|
|
|
|
|
|
|
|
|
|||
Foreign |
|
|
|
|
|
|
|
|
— |
|
||
Total Current |
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
|
|
|
|
|
|
|
|
|
|||
Deferred: |
|
|
|
|
|
|
|
|
|
|||
Federal |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
State |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
Foreign |
|
|
|
|
|
— |
|
|
|
— |
|
|
Total Deferred |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
|
|
|
|
|
|
|
|
|
|||
Total Income Tax Provision |
|
$ |
|
|
$ |
( |
) |
|
$ |
|
||
The following table reconciles the expected statutory federal income tax to the actual income tax provision:
|
December 31, 2023 |
|
December 31, 2022 |
|
||||||||||||
|
|
Amount |
|
|
Percent |
|
|
Amount |
|
|
Percent |
|
||||
Net (loss) income before taxes |
|
$ |
( |
) |
|
|
|
|
$ |
( |
) |
|
|
|
||
Expected income benefit at statutory tax rate |
|
|
( |
) |
|
|
% |
|
|
( |
) |
|
|
% |
||
IRC 280E adjustment |
|
|
|
|
|
- |
% |
|
|
|
|
|
- |
% |
||
Return to provision true-up |
|
|
|
|
|
- |
% |
|
|
( |
) |
|
|
% |
||
Impairment of goodwill and intangible assets |
|
|
|
|
|
- |
% |
|
|
|
|
|
- |
% |
||
Changes in unrecognized tax benefits |
|
|
|
|
|
- |
% |
|
|
|
|
|
- |
% |
||
Extinguishment of debt |
|
|
|
|
|
- |
% |
|
|
( |
) |
|
|
% |
||
Canada income taxes at different statutory rates |
|
|
( |
) |
|
|
% |
|
|
( |
) |
|
|
% |
||
Share based compensation |
|
|
|
|
|
- |
% |
|
|
|
|
|
- |
% |
||
Changes in valuation allowance |
|
|
|
|
|
- |
% |
|
|
|
|
|
- |
% |
||
U.S. state income taxes |
|
|
( |
) |
|
|
% |
|
|
( |
) |
|
|
% |
||
Revaluation of equity/warrants |
|
|
( |
) |
|
|
% |
|
|
( |
) |
|
|
% |
||
Revaluation of contingent consideration |
|
|
( |
) |
|
|
% |
|
|
( |
) |
|
|
% |
||
Other |
|
|
( |
) |
|
|
% |
|
|
( |
) |
|
|
% |
||
Actual income tax provision |
|
$ |
|
|
|
- |
% |
|
$ |
( |
) |
|
|
% |
||
As the operations of the Company are predominantly U.S. based, the Company has prepared the tax rate table using the U.S. Federal tax rate of
F-49
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
The following table presents a reconciliation of unrecognized tax benefits:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Balance at beginning of year |
|
$ |
|
|
$ |
|
||
Increases based on tax positions related to current periods |
|
|
|
|
|
|
||
Increase (decrease) based on tax positions related to prior periods |
|
|
|
|
|
|
||
Decreases related to settlements with taxing authorities |
|
|
( |
) |
|
|
( |
) |
Balance at end of year |
|
$ |
|
|
$ |
|
||
Interest and penalties related to unrecognized tax benefits are recorded as components of the provision for income taxes. The Company had $
The increase in uncertain tax positions is primarily due to legal interpretations that challenge the Company's tax liability under Section 280E of the Code (“280E Tax Position”). As a result, the Company has reclassified approximately $
The Company's unrecognized tax benefits, inclusive of accruals for income tax related penalties and interest, include $
The principal component of deferred taxes are as follows:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Deferred tax assets |
|
|
|
|
|
|
||
Net operating losses |
|
$ |
|
|
$ |
|
||
Reserves |
|
|
|
|
|
|
||
Share issuance costs |
|
|
|
|
|
— |
|
|
Property and equipment |
|
|
— |
|
|
|
|
|
Intangible assets |
|
|
|
|
|
|
||
Other |
|
|
|
|
|
|
||
Total deferred tax assets |
|
|
|
|
|
|
||
Valuation allowance |
|
|
( |
) |
|
|
( |
) |
Net deferred tax assets |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Deferred tax liabilities |
|
|
|
|
|
|
||
Intangible assets |
|
$ |
( |
) |
|
$ |
( |
) |
Property and equipment |
|
|
( |
) |
|
|
— |
|
Total deferred tax liabilities |
|
$ |
( |
) |
|
$ |
( |
) |
|
|
|
|
|
|
|
||
Net deferred tax liabilities |
|
$ |
( |
) |
|
$ |
( |
) |
The Company assesses available positive and negative evidence to estimate if it is more likely than not to use certain jurisdiction-based deferred tax assets including net operating loss carryovers. On the basis of this assessment, the Company continues to maintain a valuation allowance on certain deferred tax assets for the year ended December 31, 2023.
As of December 31, 2023, the Company had $
F-50
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
that expire at different times. The statute of limitations with respect to our federal returns remains open for tax years 2020 and forward. Certain acquired subsidiaries are under IRS audit for tax years ended September 30, 2014 and September 30, 2015, in which upon acquisition, the seller set aside cash in an escrow account to be used on future tax indemnifications. As of December 31, 2023, the Company recorded an indemnification asset of $
As the Company operates in the cannabis industry, it is subject to the limitations of Section 280E of the Code, under which the Company is only allowed to deduct expenses directly related to sales of product. This results in permanent differences between ordinary and necessary business expenses deemed non-deductible under Section 280E of the Code. Therefore, the effective tax rate can be highly variable and may not necessarily correlate with pre-tax income or loss.
Related to its 280E Tax Position, the Company has begun the process of amending its 2020, 2021 and 2022 tax returns to reduce the income tax paid, which is reflected as an unrecognized tax benefit. The Company’s tax returns remain subject to examination by the US Federal, US State and non US taxing authorities for years ending December 31, 2019 and forward.
18. General and administrative expenses
The Company’s general and administrative expenses were as follows:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Office and general |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Professional fees |
|
|
|
|
|
|
|
|
|
|||
Lease expense |
|
|
|
|
|
|
|
|
|
|||
Facility and maintenance |
|
|
|
|
|
|
|
|
|
|||
Salaries and wages |
|
|
|
|
|
|
|
|
|
|||
Share-based compensation |
|
|
|
|
|
|
|
|
|
|||
Sales and marketing |
|
|
|
|
|
|
|
|
|
|||
Total |
|
$ |
|
|
$ |
|
|
$ |
|
|||
19. Revenue, net
The Company’s disaggregated revenue by source, primarily due to the Company’s contracts with its external customers were as follows:
|
|
|
||||||||||
|
For the Twelve Months Ended |
|
|
|
|
|||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Retail |
|
$ |
|
|
$ |
|
|
|
|
|||
Wholesale |
|
|
|
|
|
|
|
$ |
|
|||
Total |
|
$ |
|
|
$ |
|
|
$ |
|
|||
For the years ended December 31, 2023, 2022, and 2021, the Company did
As a result of the vape recall in Pennsylvania (refer to Note 6), the Company recorded sales returns of $
F-51
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
20. Finance and other expense
Finance and other expenses were as follows:
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|||
|
For the Twelve Months Ended |
|
||||||||||
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
Interest and accretion |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Indemnification asset release |
|
|
— |
|
|
|
|
|
|
|
||
Forgiveness of principal and interest on loans |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Employee retention credits and transfer fee |
|
|
|
|
|
( |
) |
|
|
— |
|
|
Debt modification fees |
|
|
— |
|
|
|
|
|
|
— |
|
|
Other income |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Total |
|
$ |
|
|
$ |
|
|
$ |
|
|||
The indemnification asset release is the reduction of the indemnification asset related to the expiration of the escrow agreement related to the acquisition of The Apothecarium. The debt modification fees relate to amounts paid to modify the Gage Amended Term Loan which did not meet the criteria to capitalize under ASC 470, Debt.
Refer to Note 4, for further explanation about employee retention credits transfer with recourse.
21. Segment information
Operating Segment
The Company determines its operating segments according to how the business activities are managed and evaluated by the Company’s chief operating decision maker. The Company operates under
Geography
The Company has subsidiaries located in Canada and the United States. For each of the twelve months ended December 31, 2023 and 2022, net revenue was primarily generated from sales in the United States. As a result of the Reorganization (Note 3), the Company consolidated its retail location in Canada and generated $
The Company had non-current assets by geography of:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
United States |
|
$ |
|
|
$ |
|
||
Canada |
|
|
|
|
|
|
||
Total |
|
$ |
|
|
$ |
|
||
22. Capital management
The Company’s objective in managing capital is to ensure a sufficient liquidity position to safeguard the Company’s ability to continue as a going concern in order to provide returns for shareholders and benefits for other stakeholders. In order to achieve this objective, the Company prepares a capital budget to manage its capital structure. The Company defines capital as borrowings, equity comprised of issued share capital, share-based payments, accumulated deficit, as well as funds borrowed from related parties.
F-52
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
Since inception, the Company has primarily financed its liquidity needs through the issuance of share capital and debt. The equity issuances are outlined in Note 13, debt modifications are outlined in Note 10, and debt financings are outlined in Note 12.
The Company is subject to financial covenants as a result of its loans payable with various lenders. The Company was in compliance with its debt covenants as of December 31, 2023. In the event that, in future periods, the Company’s financial results are below levels required to maintain compliance with any of its covenants, the Company will assess and undertake appropriate corrective initiatives with a view to allowing it to continue to comply with its covenants. Other than these items related to loans payable, the Company is not subject to externally imposed capital requirements.
23. Financial instruments and risk management
Assets and liabilities measured at fair value
Financial instruments recorded at fair value are estimated by applying a fair value hierarchy, which prioritizes the inputs used to measure fair value into three levels and bases the categorization within the hierarchy upon the lowest level of input that is available and significant to the fair value measurement. The hierarchy is summarized as follows:
Level 1- quoted prices (unadjusted) in active markets for identical assets and liabilities
Level 2- inputs other than quoted prices that are observable for the asset or liability, either directly (prices) or indirectly (derived from prices) from observable market data
Level 3- inputs for assets and liabilities not based upon observable market data
The following table represents the fair value amounts of financial assets and financial liabilities measured at estimated fair value on a recurring basis:
|
At December 31, 2023 |
|
At December 31, 2022 |
|
||||||||||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
||||||
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Cash and cash equivalents |
|
$ |
|
|
|
— |
|
|
|
— |
|
|
$ |
|
|
|
— |
|
|
|
— |
|
||
Restricted cash |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
||
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Total Assets |
|
$ |
|
|
|
— |
|
|
|
— |
|
|
$ |
|
|
|
— |
|
|
$ |
|
|||
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Contingent consideration payable |
|
|
— |
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|||
Total Liabilities |
|
$ |
- |
|
|
$ |
|
|
$ |
|
|
$ |
- |
|
|
$ |
|
|
$ |
|
||||
There were
The valuation approaches and key inputs for each category of assets or liabilities that are classified within Level 1, Level 2 and Level 3 of the fair value hierarchy are presented below.
Level 1
Cash, cash equivalents, and restricted cash, net accounts receivable, accounts payable and accrued liabilities, and other current receivables and payables represent financial instruments for which the carrying amount approximates fair value due to their short-term maturities.
F-53
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
Level 2
The Company's derivative liability consists of a detachable warrant liability issued through the private placement (Note 13) and a conversion option related to the convertible debenture offering (Note 12).
The following table summarizes the changes in the derivative liability:
Balance at December 31, 2021 |
|
$ |
|
|
Addition on acquisition |
|
|
|
|
Fair value gain on revaluation of warrants |
|
|
( |
) |
Exercises |
|
|
( |
) |
Balance at December 31, 2022 |
|
$ |
|
|
Conversion option issued in 2023 private placement |
|
|
|
|
Detachable warrants issued in 2023 private placement |
|
|
|
|
Fair value gain on revaluation of warrants and conversion option |
|
|
( |
) |
Effects of movements in foreign exchange |
|
|
|
|
Balance at December 31, 2023 |
|
$ |
|
The warrant liability is remeasured each period using the Black-Scholes Model. The Company recognized a gain on fair value of warrants of $
The Gage warrant liability was remeasured to fair value at December 31, 2022 and expired during the fourth quarter of 2023.
Series A, B, C, and D convertible preferred stock issued through its 2020 private placements expired during the second quarter of 2023.
Detachable Warrants
The detachable warrants issued as a part of the June 2023 private placement (Note 13) have been measured at fair value as of December 31, 2023. Key inputs and assumptions used in the Black-Scholes Model were as follows:
|
|
December 31, 2023 |
|
|
June 30, 2023 |
|
||
Common Stock Price of TerrAscend Corp. |
|
$ |
|
|
$ |
|
||
Option exercise price |
|
$ |
|
|
$ |
|
||
Annual volatility |
|
|
% |
|
|
|||
Annual risk-free rate |
|
|
% |
|
|
|||
Expected term (in years) |
|
|
|
|
|
|||
Bifurcated conversion options
The conversion option issued as a part of the June 2023 private placement (Note 12) have been measured at fair value as of December 31, 2023. Key inputs and assumptions used in the Black-Scholes Model were as follows:
|
|
December 31, 2023 |
|
|
June 30, 2023 |
|
||
Common Stock Price of TerrAscend Corp. |
|
$ |
|
|
$ |
|
||
Option exercise price |
|
$ |
|
|
$ |
|
||
Annual volatility |
|
|
% |
|
|
|||
Annual risk-free rate |
|
|
% |
|
|
|||
Expected term (in years) |
|
|
|
|
|
|||
F-54
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
The conversion option issued as a part of the August 2023 private placement (Note 12) have been measured at fair value as of December 31, 2023. Key inputs and assumptions used in the Black-Scholes Model were as follows:
|
|
December 31, 2023 |
|
|
August 2, 2023 |
|
||
Common Stock Price of TerrAscend Corp. |
|
$ |
|
|
$ |
|
||
Option exercise price |
|
$ |
|
|
$ |
|
||
Annual volatility |
|
|
% |
|
|
% |
||
Annual risk-free rate |
|
|
% |
|
|
% |
||
Expected term (in years) |
|
|
|
|
|
|
||
Level 3
The purchase option derivative asset expired during the second quarter of 2023.
Contingent Consideration Payable
The fair value of the Peninsula Contingent Consideration was calculated using the Black-Scholes Model. Key inputs and assumptions were as follows:
|
|
December 31, 2023 |
|
|
June 28, 2023 |
|
||
Common Stock Price of TerrAscend Corp. |
|
$ |
|
|
$ |
|
||
Option exercise price |
|
$ |
|
|
$ |
|
||
Annual volatility |
|
|
% |
|
|
% |
||
Annual risk-free rate |
|
|
% |
|
|
% |
||
Expected term (in years) |
|
|
|
|
|
|
||
As a result of the revaluation, the Company recognized a gain on revaluation of contingent consideration of $
Risk Management
The Company’s risk exposure and the impact on the Company’s financial instruments are summarized below:
(a) Credit risk
Credit risk is the risk of financial loss to the Company if a customer or counterparty to a financial instrument fails to meet its contractual obligations. Financial instruments that potentially subject the Company to significant concentrations of credit risk consist principally of cash and cash equivalents, and accounts receivable, net. The Company assesses the credit risk of trade receivables by evaluating the aging of trade receivables based on the invoice date. The carrying amounts of trade receivables are reduced through the use of an allowance account and the amount of the loss is recognized in the Consolidated Statements of Operations and Comprehensive Income (Loss). When a trade receivable balance is considered uncollectible, it is written off against the allowance for expected credit losses.
Subsequent recoveries of amounts previously written off are credited against operating expenses in the Consolidated Statements of Operations and Comprehensive Income (Loss). The Company regularly monitors credit risk exposure and takes steps to mitigate the likelihood of these exposures resulting in actual loss. The Company had
F-55
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
(b) Liquidity risk
The Company is exposed to liquidity risk, or the risk that the Company will not be able to meet its financial obligations as they become due. The Company manages liquidity risk through ongoing review of its capital requirements. The Company’s objective with respect to its capital management is to ensure it has sufficient cash resources to maintain its ongoing operations.
(c) Market Risk
The significant market risk exposures to which the Company is exposed are foreign currency risk and interest rate risk.
Foreign currency risk is the risk that a variation in exchange rates between the Canadian dollar and U.S. dollar and other foreign currencies will affect the Company’s operations and financial results.
The Company holds cash and cash equivalents in currencies other than their functional currency. The Company does not currently engage in currency hedging activities to limit the risks of currency fluctuations. Consequently, fluctuations in foreign currencies could have a negative impact on the profitability of the Company's operations. However, as of the year ended December 31, 2023, a
Interest rate risk is the risk that future cash flows will fluctuate as a result of changes in market interest rates. In respect of financial assets, the Company's policy is to invest excess cash at floating rates of interest in cash equivalents, in order to maintain liquidity, while achieving a satisfactory return.
Fluctuations in interest rates impact the value of cash equivalents. The Company’s investments in guaranteed investment certificates bear a fixed rate and are cashable at any time prior to maturity date.
The Company does not have significant cash equivalents for the year ended December 31, 2023. The Chicago Atlantic Term Loan, Pelorus Term Loan, and Stearns Loan, have variable interest rates that are tied to the U.S. "prime rate" and SOFR. At December 31, 2023, a
24. Commitments and contingencies
Legal proceedings
In the ordinary course of business, the Company is involved in a number of lawsuits incidental to its business, including litigation related to intellectual property, product liability, employment, and commercial matters. Although it is difficult to predict the ultimate outcome of these matters, management believes that any ultimate liability would not have a material adverse effect on the Company’s Consolidated Balance Sheets or Consolidated Statements of Operations and Comprehensive Income (Loss). Other than as set out below, at December 31, 2023, there were no pending lawsuits that could reasonably be expected to have a material effect on the results of the Company’s Consolidated Financial Statements, except for the proceedings described below.
Pure X Litigation
On August 9, 2023, AEY Capital LLC (“AEY”), a licensed subsidiary of TerrAscend, filed a lawsuit in Oakland County Circuit Court (the "Oakland Court") against Pure X, LLC (“Pure X”) seeking damages in the amount of $
F-56
TERRASCEND CORP
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except per share/unit amounts)
25. Subsequent events
On January 2, 2024, the Company completed a prepayment of the Ilera Term Loan of $
On January 15, 2024, the Company paid off the IHC Real Estate LP promissory note with a payment of $
On January 19, 2024, the Company acquired the remaining
F-57